Chapter 2
Risk analysis processes for the importation of beef and beef products
2.1
On 20 October 2009 the Australian government announced a new policy,
effective from 1 March 2010, which permits the importation and/or sale of
certain beef and beef products under agreed conditions from countries that have
reported cases of Bovine Spongiform Encephalopathy (BSE). The policy is set out
in Bovine Spongiform Encephalopathy (BSE): Requirements for the Importation
of Beef and Beef Products for Human Consumption – effective March 2010.[1]
2.2
In its first report the committee expressed the view that the decision
to relax the import requirements for beef and beef products should have been
preceded by a formal analysis of the import risk attached to such products. While
noting the review of current scientific evidence undertaken by Professor John
Mathews, the committee expressed concern that there had not been a greater
attempt to confirm that our current understanding of the animal health risks
posed by BSE within the current global regulatory environment is accurate. The
committee also expressed concern that a thorough consideration of all likely
consequences of an incursion of BSE, including the economic consequences, had
not been undertaken prior to the decision to relax the policy.
2.3
The committee recommended that this policy and all administrative processes
for the assessment of applications from countries seeking to import beef and/or
beef products be suspended pending the outcome of a formal overarching import
risk analysis modelled on the expanded import risk analysis process provided
for in the Import Risk Analysis Handbook 2007 (update 2009).[2]
2.4
On 8 March 2010, the Minister for Agriculture, Fisheries and Forestry
announced that he had written to the Secretary of the Department of
Agriculture, Fisheries and Forestry to request that Biosecurity Australia (BA)
conduct an Import Risk Analysis (IRA) on the importation of fresh beef and beef
products (chilled or frozen) for human consumption from countries other than
New Zealand. The Minister said:
There are three differences between the decision I have taken
today and the process available since March 1.
This is a formal review process with specified time lines,
guaranteed opportunities for community engagement and consultation as well as
the added assurance of review by the Eminent Scientists Group.[3]
2.5
Prior to this announcement, BA had announced that it would consider
market access requests on a country-by-country basis through an import risk
analysis conducted inline with the Import Risk Analysis Handbook, but outside
the regulated IRA process.[4]
Assessment by the Australian BSE Food Safety Assessment Committee
2.6
Under the policy which came into effect on 1 March 2010, countries
wishing to export beef and beef product to Australia for human consumption can
apply for a country assessment and will be assigned one of three categories:
- Category 1 – Countries assessed by Australia as meeting
the ‘Negligible BSE Risk’ requirements of the Terrestrial Animal Health Code of
the World Organisation for Animal Health (OIE). Beef and beef products can be
imported subject to specific requirements.
- Category 2 – Countries assessed by Australia as meeting
the ‘Controlled BSE Risk’ requirements of the Terrestrial Animal Health Code of
the World Organisation for Animal Health (OIE). Beef and beef products can be
imported subject to specific requirements.
- Category 3 – Countries assessed by Australia that do not
meet the requirements of either Category 1 or Category 2, or countries that
have not applied to be assessed by Australia. Beef and beef products cannot be
imported.
2.7
The committee considered this risk assessment process in its first
report.[5]
It noted that under the new policy, countries will submit a completed
Australian Questionnaire to Assess BSE Risk. The risk assessment will be
administered by FSANZ, based on the risk assessment methodology developed by
the World Organisation for Animal Health (OIE) and will be reviewed by the
Australian BSE Food Safety Assessment Committee. The assessment may involve an
in-country inspection to verify in-country control measures.
2.8
Each final assessment report will be submitted to the Chief Executive
Officer of FSANZ for consideration. Subject to approval, the final report will
be provided to the Deputy Secretary of the Biosecurity Services Group within
the Department of Agriculture, Fisheries and Forestry and also to the applicant
country.
2.9
The following flowchart sets out the assessment and certification
process FSANZ will follow upon receipt of an application.
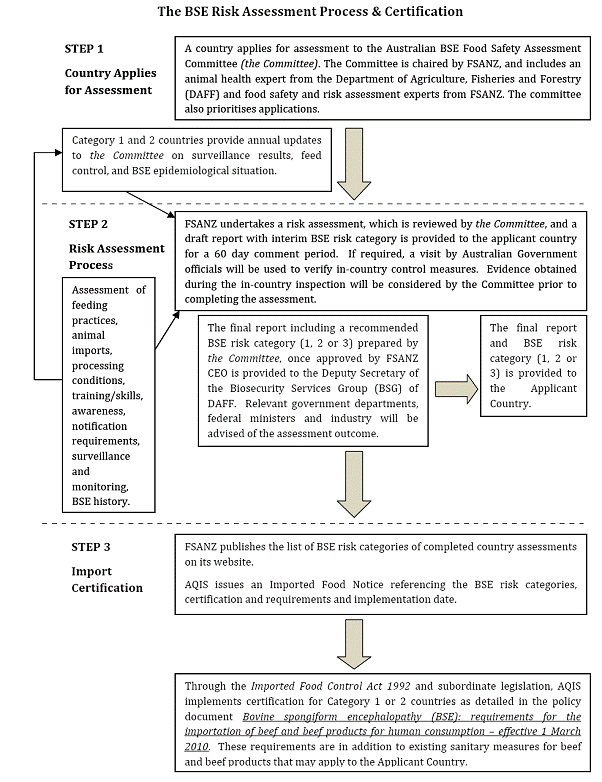
2.10
This process provides for a draft report, including an interim
recommended BSE risk category, to be provided to the applicant country for
comment. The process does not include provision for public consultation and
there is no requirement for publication of the final assessment reports. However,
the final BSE risk category of all completed country assessments, other than
those categorised as Category 3, will be published on the FSANZ website. [6]
2.11
The committee notes that FSANZ anticipates the assessment process will
take an average of 20 weeks to complete from the time of application to the
preparation of a draft report, depending on the quality and completeness of the
information provided in the application and whether an in-country inspection is
required.[7]
2.12
The committee also notes that during the 2010-2011 Budget Estimates
hearings the Rural Regional Affairs and Transport Legislation Committee (the
Legislation committee) was advised that:
It is still the case today that we have not had an
application through to FSANZ from either the United States or Canada, but I
understand one is imminent from Canada. We in Biosecurity Australia have
accepted applications through letters that we had earlier from the United
States and Canada, so our clock started ticking on 8 April to undertake the
IRA. In that regard it has started, but we have not had an application from the
United States through to FSANZ, so the planning for an in-country inspection of
specific locations from which they wish to export has not been determined at
this stage.[8]
2.13
Under the new BSE food safety policy for imported beef and beef
products, countries currently allowed to trade beef products with Australia are
required to submit an application to FSANZ for BSE risk status by 30 June 2011
to continue to export beef and beef products to Australia after 1 July 2011.
2.14
Mr Steve McCutcheon advised the committee at its 14 May 2010 hearing
that one application had been lodged (by New Zealand) on 28 April 2010.
On 28 April 2010 FSANZ received an application from the New
Zealand government for FSANZ to undertake a food safety risk assessment and
assess its BSE risk status under the Australian government’s new BSE food
safety policy for imported beef and beef products. New Zealand is one of a
number of countries currently allowed to trade beef products with Australia.
Under the new BSE food safety policy, such countries are required to submit an
application to FSANZ for BSE risk status by 30 June 2011 to continue to export
beef and beef products to Australia after 1 July 2011. Public notification of
the receipt of this application was made on 7 May 2010. This is the first and
only application FSANZ has received since the new BSE food safety policy came
into effect on 1 March 2010. [9]
Import risk analysis by Biosecurity Australia
2.15
On 8 April 2010 Biosecurity Australia announced the formal commencement
of separate concurrent import risk analyses to assess the animal quarantine
risks from the importation of beef and beef products intended for human
consumption from the United States, Canada and Japan.[10]
These IRA processes are being conducted in parallel with the BSE food safety
risk assessment being undertaken by FSANZ.
2.16
The IRA processes will be led by Dr Mike Nunn, Principal Scientist,
Animal Biosecurity and an expert panel has been established to assist in their
conduct. The expert panel comprises:
- Associate Professor John Glastonbury, Associate Professor of
Diagnostic Pathology, School of Animal and Veterinary Sciences, Charles Sturt
University;
- Dr Kevin Doyle, National Veterinary Director, Australian Veterinary
Association; and
- Dr Ron Glanville, Chief Veterinary Officer, Queensland.[11]
2.17
The IRAs will be completed within 24 months from the initial BA announcement.
BA will prepare draft IRA reports which will be circulated to stakeholders who
will have up to 60 days to provide written comments.[12]
2.18
The draft IRA reports will:
- confirm the pests and diseases being assessed within the IRA and describe
the major pathways for entry, establishment and spread in Australia;
- determine the likelihood of entry, establishment or spread and
the harm that could result;
- specify whether the resulting risks exceed Australia's appropriate
level of protection (ALOP) and where the risks exceed Australia's ALOP,
identify potential risk management measures and determine whether application
of the measures could reduce the risks to achieve Australia's ALOP; and include
a preliminary view of the preferred options for risk management.[13]
2.19
The following flowchart sets out the Import Risk Analysis process.
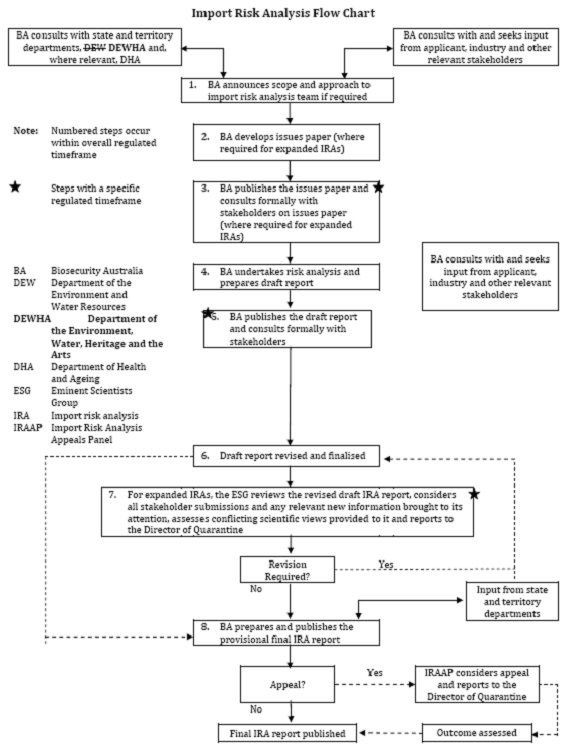
Stakeholder consultation
2.20
As noted above, the process outlined in the IRA Handbook 2007 (update
2009) (the handbook), allows for formal consultation with stakeholders
following completion of the draft IRA. The Quarantine Regulations 2000 provide
that this consultation period must be no longer that 60 days from and including
the day the invitation to comment is published.[14]
However, the committee notes that in the event that the Chief Executive BA
considers that stakeholders may not have had reasonable opportunity to comment
on the draft IRA report, the Chief Executive has the discretion to extend the
consultation period by up to 60 days.[15] The IRA Handbook also
indicates that BA may also meet with stakeholders to discuss the draft IRA
reports.[16]
2.21
The committee notes that the public file of non-confidential submissions
and other documentation, together with information regarding the status of each
IRA, is available on BAs website and that BA maintains a data base of
registered stakeholders to facilitate engagement and communication with people
and organisations with an interest in the IRA throughout the process.
2.22
The committee insists that the draft IRA report be available for
public/stakeholder scrutiny, including by the Parliament and this committee, at
the commencement of the stipulated consultation period.
Parliamentary scrutiny of risk assessment processes
2.23
In its first report, the committee expressed concerns regarding the lack
of Ministerial and Parliamentary scrutiny of decisions taken under the new
policy for the importation of beef and beef products. The committee noted that
the categorisation of applicant countries will be undertaken by FSANZ on behalf
of the Australian BSE Food Safety Committee (ABFSA) and will be approved by the
Chief Executive Officer of FSANZ. Similarly, the committee noted that any
reviews of country classifications will be considered by ABFSA and any
subsequent review of the policy or the questionnaire through which it is
primarily administered will be undertaken at the discretion of FSANZ.[17]
2.24
The committee has expressed concerns in previous inquiries about a
similar lack of Ministerial and Parliamentary scrutiny in relation to the IRA
process administered by BA[18]
The committee notes that the completion of an IRA process is signalled by the
determination of the policy framework against which decisions to grant import
permits, and the extent to which conditions may be attached to a permit will be
made. The committee notes that this determination is made by the Director of
Animal and Plant Quarantine and that this position is held by the Secretary, Department
of Agriculture, Fisheries and Forestry.
2.25
In making the determination the Director of Animal and Plant Quarantine
considers:
- the final IRA report and its recommendations;
- the outcome of any appeals;
-
the report of the ESG;
- BA's response to the ESG report; and
- any other relevant information, including Australia's
international rights and obligations.
2.26
The Director of Animal and Plant Quarantine notifies AQIS and BA of the
policy determination and BA, in turn, notifies the World Trade Organisation
Secretariat. The committee notes that this policy determination is not approved
by the Minister and is not subject to Parliamentary scrutiny. The committee
also notes that the only avenue for review in relation to this policy decision
appears to be judicial review under the Administrative Decisions (Judicial
Review) Act 1977 in relation to an individual import permit decision taken
by the Director of Animal and Plant Quarantine.
2.27
As noted in its first report, the committee accepts that the officers of
FSANZ and BA are ultimately accountable to the relevant Minister. However, the
committee does not accept that this is the same as Ministerial sign off on
policy decisions, or parliamentary scrutiny of significant changes in policy.
Recommendation 1
2.28 The committee recommends a process whereby the relevant Minister is
required to consider and rule on the recommendations provided by Biosecurity
Australia, following an Import Risk Analysis, and the Australian BSE Food
Safety Assessment Committee, following a country assessment. The committee also
recommends that the relevant Minister report any decision to approve or reject
such recommendations to the Parliament and this committee prior to a determination
by the Director of Plant and Animal Quarantine, in the case of an Import Risk
Analysis, or the Chief Executive Officer of FSANZ, in the case of a country
assessment, and prior to formal advice being provided to the applicant country.
'Stop the clock' provision invoked
for IRA for beef from Japan
2.29
On 10 May 2010, BA announced that it had invoked the 'stop the clock'
provision for the IRA for the importation of beef and beef product for human
consumption from Japan following confirmation of an outbreak of foot and mouth
disease (FMD) in cattle in Japan. On 20 April 2010 the Japanese Ministry of
Agriculture, Forestry and Fisheries advised the detection of FMD in breeding
cattle at a farm in Miyazaki Prefecture of Kyushu Island. FMD is internationally
recognised as a very serious disease of cloven hoofed animals and Australia has
strict quarantine measures for FMD. These measures include not permitting the
importation of fresh beef and beef products from any country affected with FMD.[19]
2.30
Under Clause 69H of the Quarantine Regulations 2000, certain
periods of time in the IRA process may be disregarded, such as where a
significant international quarantine circumstance exists that limits BA's
ability to complete an IRA. Until Japan regains its former FMD-free status
according to the World Organisation for Animal Health and following an
evaluation by Australia, any time that elapses will not be considered as being
within the regulated deadline for the IRA.[20]
2.31
The committee sought clarification from BA regarding the impact of any
past or future diagnosed cases of BSE within the United States or Canada on the
IRA processes in relation to those countries. The committee asked whether such
cases might trigger the 'stop the clock' provisions. Dr Andrew Carroll told the
committee:
In relation to Canada, the answer is no. The slightly longer
answer is that given their current BSE categorisation of status, there would be
an expectation that they would from time to time have a case that would be
taken into consideration as part of the process that they have gone through.[21]
2.32
Dr Carroll went on to state that it is not beyond BA's expectation that
Canada or the United States could have another case of BSE.[22]
Dr Colin Grant, Chief Executive, Biosecurity Australia, explained to the
committee that the IRA process was intended to assess the conditions under
which product from these countries can come into Australia.[23]
Dr Grant contrasted this with products from countries with diagnosed cases of
FMD. Dr Grant told the committee:
Dr Grant—Products from countries with
foot-and-mouth-disease cannot enter this country—
Senator BACK—Fresh?
Dr Grant—Fresh, and that is the end of that until such
times as they are clear of that and we have reassessed the OIE assessment of
their clearance. The exercise in terms of Canada is, in fact, to do precisely
what you identified and that is to assess the conditions under which their
product can come into Australia.[24]
2.33
The committee finds Dr Carroll's statement regarding BA's expectations
regarding Canada and the United States of concern. The committee believes that
where there is an expectation that a country may continue to report cases of
BSE, this should be a key consideration in the development of the IRA for that
country.
Independent review by the Eminent
Scientists Group
2.34
BA's 8 April 2010 announcement states that the IRA will be a standard
IRA. The committee notes that the Import Risk Analysis Handbook distinguishes
between standard and expanded IRA processes and that a key difference between a
standard and an expanded IRA is the provision for Review by the Eminent
Scientists Group (ESG).[25]
2.35
However, the committee notes that in announcing his decision to request
an IRA, the Minister stated that the IRA process would provide the added
assurance of review by the Eminent Scientists Group. BA has also confirmed that
the independent Eminent Scientists Group will provide external scientific
scrutiny of the IRA reports after consideration of public comments.[26]
2.36
The committee welcomes the inclusion of high level review by the ESG,
independent of BA. The committee notes that following the independent review of
Australia's quarantine and biosecurity system in 2008, chaired by Roger Beale
AO, the role of the ESG was expanded to include review of whether all relevant
matters relating to the likely economic consequences of a pest or disease
incursion have been properly considered. Mr Roger Rose, former Chief Economist
at the Australian Bureau of Agricultural and Resource Economics was appointed
to the ESG from 1 July 2009.[27]
2.37
The ESG is now tasked with providing external scientific and economic
scrutiny of IRAs.[28]
The committee notes that the scope of the ESG's role, as set out in the IRA
Handbook, is relatively wide. In performing its role the ESG is to:
take account of any relevant new information brought to its
attention and assess conflicting scientific views to ensure that:
- all submissions received from
stakeholders in response to the draft IRA report have been properly considered;
- all relevant matters relating to
the likely economic consequences of a pest or disease incursion have been
properly considered; and
- the conclusions of the revised
draft IRA report are scientifically reasonable, based on the material
presented.[29]
2.38
The committee notes that the ESG may consult with Biosecurity Australia
and with stakeholders and that the Chairman of the ESG may co-opt additional
expertise or seek advice to assist the ESG in meeting its terms of reference.
However, the committee notes that the ESG must complete its review within a
maximum timeframe of 60 days. The ESG report will be provided to the Chief
Executive of BA and will be publicly released.
[30]
Development of effective import protocols
2.39
In its first report, the committee noted the fundamental importance of
the development of effective import protocols for the importation of beef and
beef products. The committee expressed concern that such protocols should be
developed in close consultation with the industry to ensure that all concerns
are considered and appropriately addressed.
2.40
The committee notes that the IRA process administered by BA provides
opportunities for stakeholders to comment on the analysis of risk and the
possible measures which might be applied to effectively manage these risks. Dr
Grant told the committee:
There will be a process of assessment jointly done by FSANZ
and Biosecurity Australia. The documentation for all of that in the context of
the import risk analysis, the IRA, that is done will be put out for public
comment for 60 days and the public, in all its guises, will be able to assess
what we have done in that entire risk assessment process, which will include
in-country inspections and an assessment of the systems.[31]
2.41
In its first report the committee noted assurances that applicant
countries will be required to demonstrate equivalence with the requirements
currently applying to Australia's own beef industry. In particular, the
committee noted that there is a clear expectation within the beef industry, BA,
FSANZ and among government ministers that applicant countries will be required
to meet equivalent traceability standards to those applied to Australian beef
producers.[32]
2.42
The committee notes that the country assessments undertaken by FSANZ provide
for examination of animal traceability and identification systems within the
applicant country. Applicant countries are required to provide the following
documentary evidence to demonstrate the systems the country has in place to
ensure the effective and timely identification and tracing of potentially BSE
infected cattle, their birth and feed cohorts:
- Documentation of the herd identification systems in the country,
including any relevant legislation and/or industry standards.
- Documentation of the process and timeframe whereby cattle at
slaughter that are suspected to be BSE positive can be identified and traced
back to the farm of origin and farms of residence.
- Documentation of the process and timeframe whereby cattle from
the same birth or feed cohort to the BSE positive cases can be identified and
traced forward to the point of slaughter, death or residence.
- Documentation of the risk management of cattle suspected to have
been exposed to feed that has been cross-contaminated with meat-and-bone meal
or greaves of bovine origin identification and traced forward to the
point of slaughter, death or residence.[33]
2.43
In its first report the committee expressed concern that it was not
clear how FSANZ would assess the equivalence of this evidence. However, the committee
notes Dr Grant's assurance that:
... without pre-empting the review itself and the assessment
process, one of the issues we will be looking at is the chain of custody of the
product and being able to be comfortable and being able to accept certification
that is able to be demonstrably evident of containment.[34]
Committee view
2.44
The committee considers that the ability to efficiently and confidently
trace product from the point of sale to both its country and farm of origin,
and to effectively identify the cohorts of the animals concerned, is
fundamental to management of the human and animal health risks associated with
the importation of beef and beef products. Throughout the inquiry the committee
has expressed concern that such traceability systems must be capable of
extending across country borders.
Recommendation 2
2.45 The committee recommends that unless there is full traceability across national
borders (equivalent to Australia's traceability requirements) both countries
must be considered to pose equal risk.
2.46
At its hearing on 14 May 2010, the committee sought to satisfy itself
that existing mechanisms enabled beef products to be traced efficiently through
the chain of production. The committee asked BA to investigate and provide the
relevant 'paper trail' of certification in relation to a sample of beef product
purchased in an Australian supermarket and labelled as Product of the United
States of America. In particular, the committee wished to see the assurances
upon which relevant quarantine officials satisfied themselves that the product
did not contain US beef and that therefore they could confidently issue an
import permit.
2.47
BA undertook to look into the committee's request but had not provided a
response to the committee at the time of tabling this report.
In-country inspections
2.48
In its first report the committee noted that the assessment process to
be undertaken by FSANZ does not provide for mandatory in-country inspections. The
committee expressed the view that in-country inspections are necessary to
ensure the adequacy of the certification and traceability systems within the
exporting country. The committee recommended that FSANZ revise the Australian
process to assess BSE risk, including the Australian Questionnaire to Assess
BSE Risk, to include a mandatory requirement for an in-country inspection to be
undertaken as part of the assessment of each application to import beef and/or
beef product to Australia.[35]
2.49
The committee notes that the BSE risk assessment process remains
unchanged. Under the risk assessment administered by FSANZ, the BSE Food Safety
Assessment Committee can initiate an in-country inspection for an applicant
country where the analysis of information provided by the applicant country, or
any other relevant information, indicates this is warranted. Verification of
in-country control measures may include examination of:
-
the existing systems in the applicant country to prevent the
spread of BSE in the cattle population and from entering the human food chain;
- the existing systems to prevent food for human consumption from
becoming contaminated during animal slaughter and processing; and
- any other relevant matter.[36]
2.50
The committee also notes that the IRA process does not require
in-country inspections. However, the committee is aware that the Agreement on
the Application of Sanitary and Phytosanitary Measures, with which Australia's
IRA process must be consistent, provides for exporting Members to give
importing Members reasonable access, upon request for inspection, testing and
other relevant procedures.[37]
The committee is also aware that in-country inspections have been undertaken as
part of previous IRA processes.
2.51
The committee notes evidence provided to the Legislation committee during
the 2010-11 Budget Estimates that suggests in-country inspections will form
part of the FSANZ and BA assessment processes for applications from Canada and
the United States and will be conducted jointly by the two agencies. The
committee is aware that planning for these inspections has not yet been
finalised. Dr Colin Grant told the Legislation committee that the planning for
in-country inspections had not commenced as applications were yet to be lodged
by Canada and the United States. Dr Grant told said:
That has not been determined at this stage. There will be, as
I understand it from FSANZ, four officers working on their risk assessment. We
have indicated that we are putting together a team of 10 to 12 to work on that
assessment. The in-country inspections will be done jointly by FSANZ and
Biosecurity Australia, or DAFF, at the time that we go over.
...
FSANZ have indicated that their process will run for the
order of six to eight months. The in-country inspections will take place during
that period; so, assuming that we will get an application reasonably soon,
sometime between now and the early part of next calendar year, and I would say
something in the order of four or five months time.[38]
Committee view
2.52
The committee is heartened by this apparent commitment to undertaking
in-country inspections as part of the assessment of import applications
received from Canada and the United States. However, the committee would prefer
to see in-country inspections included as a mandatory step in both the FSANZ
and BA risk assessment processes. In its first report the committee expressed
the view that in-country inspections must be undertaken as a matter of course
as part of the assessment of each import application. The committee stated that
it did not consider that desk top analysis was an appropriate substitute for
first hand assessment of the competencies and systems that underpin the
management of livestock prior to slaughter and export. The committee remains of
this view.
Economic impact of the new policy - consultation with Australia's current
trade partners
2.53
The Australian Beef Association (ABA) expressed concern that there
appears to have been no consultation with Australia's major export partners.
The Chairman of the ABA, Mr Brad Bellinger told the committee that the ABA was
concerned that if Australia were to allow the importation of beef from BSE
affected countries, some of Australia's major export markets may impose age
requirements on exports of Australian beef. Mr Bellinger told the committee:
As it stands with our major beef markets on the Pacific
rim—Japan, South Korea and Taiwan—Japan imposed the 20-month rule on all US
beef imports to that country and Taiwan and South Korea applied a 30-month
rule. We have concerns that if we allow beef from BSE affected countries into
Australia then these countries will then impose that rule onto our own beef
market.[39]
2.54
The ABA also expressed concern that if such requirements were imposed they
would have a significant impact on Australian beef producers. ABA Director, Mr
Athol Economou, told the committee that:
... we do not have the technical resources to segregate out the
young cattle. It has taken the Americans six years to get their act together;
it will take us decades. Meanwhile our cattle prices will collapse because
Japan and South Korea account for more than 50 per cent of our exports.[40]
2.55
Mr Hamish McCormack, First Assistant Secretary, Office of Trade
Negotiations, Department of Foreign Affairs and Trade told the committee that
all WTO members had been notified of the change in policy through the SPS
committee and that no negative issues have been raised at a government level. Dr
Carroll told the committee that:
We have had the new food safety policy out now for some six
months in the international arena, notified through the WTO, and we received no
negative feedback at all.[41]
2.56
Dr Carroll also told the committee that there appeared to have been no
adverse effect on the trade of beef from New Zealand to North Asian markets as
a result of its decision to allow beef imports from BSE affected countries and
that it was his expectation that there would be no adverse effect on Australian
beef exports.[42]
Dr Carroll explained to the committee that he did not believe Australia would
inherit the United States' status as a beef exporter as a consequence of
allowing beef from the United States to be imported into Australia. Dr Carroll
told the committee:
There is not free and open trade between us and the US. We
would not be inheriting any US status. Should it get approval, there would be
requirements applying to any product that came from the US into Australia. So
we would not inherit the US status in any way.[43]
Review of Country of Origin Labelling
2.57
In its first report the committee noted that while there are country of
origin requirements for fresh pork and seafood and fresh fruit and vegetables,
there is currently no such requirement for unpackaged fresh beef. The committee
noted that a review panel led by Dr Neal Blewett AC is currently undertaking a
comprehensive review of food labelling law and policy. The panel is due to
report the Australia New Zealand Food Regulation Ministerial Council in
December 2010 and to the Council of Australian Governments in early 2011.
2.58
Mr McCutcheon advised the committee that on 9 March 2010, the
Parliamentary Secretary for Health, the Hon. Mark Butler MP wrote to the Chair
of the FSANZ Board asking FSANZ to raise a proposal to review Standard
1.2.11 Country of Origin Requirements with a view to removing its
inconsistency in application across unpackaged meat, particularly beef.[44]
2.59
The committee notes that in announcing the review, the Parliamentary
Secretary stated that he was seeking to remove an anomaly in the country of
origin laws which apply to fresh fish and pork, but which do not include beef.
He also said:
In addition, we are seeking agreement from industry about a
new labelling system for beef products – a labelling system that will make it
easy for consumers to identify, for example, whether their meat pie contains
only Australian beef. This labelling will be enforced by the Australian
Competition and Consumer Commission.[45]
2.60
In response to this request FSANZ prepared a proposal to consider
extending country of origin labelling to unpackaged beef, lamb and chicken
meat. The committee notes that the proposal provides the following explanation
for the review:
Following changes to Australia's BSE food safety policy for
imported beef and beef products, the Parliamentary Secretary for Health has
asked FSANZ to prepare a proposal to review Standard 1.2.11 to remove its
inconsistency in application across packaged and unpackaged meat, and that
priority consideration be given to this work. This Proposal will consider
extending country of origin labelling to unpackaged beef, lamb and chicken
meat. Unpackaged pork already requires country of origin labelling. Preliminary
discussions with Industry stakeholders indicate that they are generally
receptive to extending country of origin labelling to other unpackaged meats.[46]
2.61
The committee notes that it was proposed to conduct targeted stakeholder
meetings with key stakeholders during April 2010, with the assessment to be completed
by mid June 2010. The proposal anticipates notification of the Ministerial
Council by Mid-October 2010 and gazettal in early January 2010.[47]
2.62
The committee's view is that all imported food product should be
labelled with both the country of origin and country of processing, where this
is a different country. This would provide consumers with a choice as to
whether they wish to eat beef from countries that have had a BSE outbreak. The
committee will watch the progress of the review with interest.
Recommendation 3
2.63 The committee recommends that all food product be labelled with both the
country of origin and the country of processing, if different.
Current understanding of human health risks associated with BSE
2.64
In its first report the committee noted the observation of the National
Health and Medical Research Council that there is so much about BSE and variant
Creutsfeldt-Jakob Disease that is still unknown. The committee noted the unduly
short time frame allocated to a review of the current scientific understanding
of the disease and its risks to human and animal health and expressed concern
that the resultant policy change may have been based on an incomplete
understanding of the associated risks.
2.65
At its hearing on 14 May 2010, the committee heard evidence from Dr Alan
Fahey who has recently submitted a thesis on the clinical neuropsychiatric,
epidemiological and diagnostic features of Creutzfeldt-Jakob disease.[48]
Dr Fahey told the committee of his concerns regarding the lengthy incubation
period for transmissible spongiform encephalopathies, the inadequacy of current
tests and the limited nature of our current understanding of this group of
diseases.[49]
2.66
Dr Fahey also told the committee that in the last two years a link has
been established between forms of atypical CJD and atypical BSE. Dr Fahey said
that:
They now believe that those atypical BSEs overseas are in
fact causing sporadic Creutzfeldt-Jakob disease. They were not sure if it was
due to mad sheep disease or a different form. If you look in the textbooks it
looks like this is just arising by itself. But in my research I have a summary
of a document which states that there has never been any proof that sporadic
Creutzfeldt-Jakob disease has arisen de novo—has arisen of itself. There is no
proof of that. The recent research is that in fact it is due to atypical forms
of mad cow disease which have been found across Europe, have been found in
America and have been found in Asia. These atypical forms of mad cow disease
typically have even longer incubation periods than the classical mad cow
disease.[50]
2.67
Dr Fahey suggested to the committee that there should be a government
policy requiring veterinary examination of animals that die of suspicious
diseases. Dr Fahey also suggested that post-mortem examinations and neuropathologyy
should also be performed in the case of anyone who dies with any neurological
or psychiatric condition. He said:
Post-mortem examinations and neuropathology are rarely done
on humans. I personally would like to see them mandatory for anyone dying with
any neurological or psychiatric condition. My aunt presented with psychiatric
phenomena initially, and doctors thought it was psychiatric. Psychiatric
phenomena are the way that mad cow disease presents in humans. Personally, I
think that any humans—or cattle or sheep—dying with psychiatric or neurological
disorders should have neuropathology performed.[51]
2.68
The committee reiterates its concern that understanding of this group of
diseases is currently limited but that research continues to explore new
connections between the various forms of the disease. The committee notes the
role of the National Health and Medical Research Council's Transmissible
Spongiform Encephalopathies Advisory Committee (TSEAC) in providing independent
and timely expert advice on measures necessary to prevent and limit the spread
of vCJD and other TSEs in Australia. The committee considers that TSEAC should
be formally charged with monitoring developments in the scientific
understanding of transmissible spongiform encephalopathies and the associated
risks to human and animal health and providing regular reports to the Minister for
Health and the Minister for Agriculture, Fisheries and Forestry to enable the
government to respond quickly and appropriately to new evidence as it emerges.
Conclusion
2.69
As the committee observed in its first report, the Australian beef
industry has a hard earned reputation as a disease free exporter that has
secured it entry into some of the most demanding markets in the world. The
committee can understand why some members of the industry are nervous at the
prospect of importing beef from countries that have reported cases of BSE.
2.70
In such circumstances it is essential that the assessment of
applications from countries wishing to export beef and beef products to
Australia under the relaxed policy introduced on 1 March 2010 must be thorough,
robust and transparent. The committee considers that the Minister's
announcement of a regulated IRA process for the consideration of import
applications is a significant step in this direction. While the committee has
expressed some concerns about the administration of the IRA process on other occasions,
it notes that the regulated IRA process provided for in the Import Risk
Analysis Handbook does provide key points at which stakeholders can provide comment
during the risk analysis process and includes opportunities for review and
appeal.
2.71
However, the committee remains concerned about the assessment process
administered by FSANZ. In its first report the committee noted that the
questionnaire that forms the basis of FSANZ's assessment of applications lacks
a clear statement of the criteria against which applicant countries will be
assessed and how equivalence against each of the requirements will be
determined. The committee expressed the view that greater clarity needed to be
provided in the questionnaire as to how FSANZ will be guided in its assessment
of applications. The FSANZ assessment process makes no provision for public consultation
and, while the category is published there is no requirement to publish the
assessment reports. It therefore appears that the committee and stakeholders
must be satisfied with the assurances provided throughout this inquiry that
applicant countries will be required to demonstrate equivalence with the
requirements currently applying to Australia's own beef industry.
2.72
Notwithstanding these concerns, the committee recognises that both of
these assessment processes are still at a comparatively early stage and the
committee notes that FSANZ and BA appear to be working collaboratively to
ensure an appropriate level of cooperation in relation to key areas of overlap
between the two processes. The committee notes Dr Grant's advice that the two
agencies have been working closely together:
The department will have its principal scientist on animal
biosecurity on the FSANZ BSE food safety assessment committee. Regular meetings
between FSANZ and the Biosecurity officers are being held and a steering
committee for ongoing management of the assessment processes has been
established and will be supported by a dedicated project manager.[52]
2.73
The committee is particularly pleased to note that BA and FSANZ appear
to be planning to undertake in-country inspections as part of the assessment
processes for Canada and the United States.
2.74
The committee remains concerned that the adequacy of traceability and
certification systems is paramount in the management of risks associated with
the beef from countries which have reported cases of BSE. The ability to
identify and investigate possible incursions quickly and effectively and take
appropriate action depends upon the robustness of these systems. The committee
considers that these systems must be subject to rigorous first-hand inspection,
both during the application process and on a regular on-going basis. With this
in mind, the committee intends to monitor the progress of the Import Risk
Analysis process closely and will inquire further into this matter if
necessary.
Senator Fiona Nash
Chair
Navigation: Previous Page | Contents | Next Page