The anecdotal data goes to, I think, a clear and broad and
accepted understanding that the private sector prices are, on average, too
high. But there is a risk in arbitrary price reductions that you hit the wrong
target in the wrong way and you lose products from the Australian market by
unwittingly cutting too deep in a particular area or removing some of the
current practices which involve clinical support. So, we are working in an area
of a clear understanding that there is a problem but not a thorough dataset
that provides a recipe.[1]
4.1
While all stakeholders agree that changes to the current Prostheses List
(PL) framework are needed, it appears there is no one solution, and that no
solution or suite of solutions will be agreeable to all stakeholders. As the
Chair of the Prostheses List Advisory Committee (PLAC) has stated:
'If there is an answer to this issue that you are
deliberating about, it is not going to be one answer; it is going to be
multiple things in parallel including national and international reference
prices... It is going to potentially be price disclosure if we can make that
useful. It may be a number of other things.'[2]
4.2
This chapter will focus on trying to highlight some of the key areas
identified by stakeholders where the current reforms could focus in attempting
to address the historical and ongoing concerns in relation to prostheses prices
for privately insured patients.
Do we need a Prostheses List?
4.3
A number of stakeholders to this inquiry have suggested that the PL
should be phased out over time to allow the market to determine what private
health insurers pay for prostheses. Private Healthcare Australia (PHA)
submitted that the current Prostheses List framework is 'winning the battle but
losing the war: price inflation is under control, but reimbursement levels
remain significantly higher than other comparable health systems.'[3]
4.4
In their Pre-Budget Submission 2017–18, PHA recommended that, in
addition to rapid implementation of the proposed PL reform agenda in 2017–18,
the government 'should have an explicit deadline to exit regulation of
medical device benefits in the private health sector.'[4]
4.5
The majority of submitters and witnesses, however, have argued that some
form of PL is necessary and desirable, particularly given the complex system in
which prostheses are selected, purchased, paid for and reimbursed when a
patient is privately insured.
4.6
The Department of Health (department) provided the following figure to
show the impact of deregulation and re-regulation, showing that re-regulation
coincided with stabilisation of the average benefit paid per prosthesis.
Figure 4.1: Prostheses expenditure
and average benefit per prosthesis – 1989/90 to 2015/16
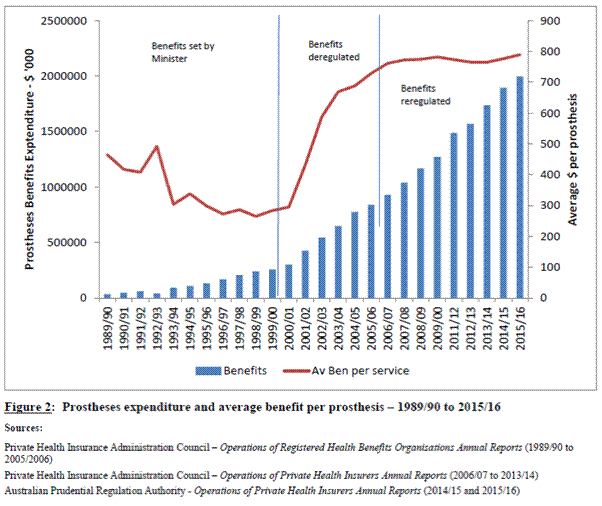
Source: Department of Health,
Submission 38, p. 11.
4.7
Ramsay Health Care Limited stated that:
The Prostheses List was introduced to address issues arising
from the previously deregulated environment, which saw rapid increases in
prostheses pricing. The Prostheses List has also resulted in additional
assurances of device safety and efficacy.[5]
4.8
Catholic Health Australia (CHA) members support the exploration of
proposals to provide a more competitive approach to benefit setting whilst
maintaining the benefit of certainty and access to technology provided by the
PL and PLAC processes:
The PL and PLAC have been instrumental in slowing the rising
costs of devices and ensuring that patients continue to have access to
appropriate and up to date technologies. CHA recommends that the PL and
existing architecture of the PLAC be maintained and incorporated into any
future model that is adopted by the Government and private health industry.[6]
4.9
All stakeholders have indicated a need for change. The degree of change,
from moves to improve administration and transparency of PLAC, the PL and
health technology assessment (HTA) processes more broadly, through to abolition
of government regulation, is where there is little consensus between
stakeholders.
Review of PL listing criteria
4.10
One of the key areas for reform of the PL framework being undertaken by
the PLAC is a review of the criteria for listing on the PL. PLAC has listed the
desired outcomes of the criteria review as:
-
Privately insured Australians have access to medical devices that
meet their healthcare needs through their private health insurance.
-
Evidence requirements for listing on the Prostheses List are
appropriate and defensible.[7]
4.11
The proposed activities to review the definition of prostheses include
comparing the current definition with those used in comparable processes and
regulatory arrangements, and to consult on any proposed changes to this
definition, including in relation to any impacts and transition arrangements.[8]
Defining prostheses
4.12
A broad range of stakeholders to this inquiry have called for a change
to the definition of prostheses used for regulation of prostheses prices in the
private sector.[9]
In its report, the Industry Working Group on Private Health Insurance Prostheses
Reform (IWG) stated that they had agreed that the current definition of
prostheses should be reviewed, particularly given 'the rapid development of
novel medical technology.'[10]
4.13
Changes to the definition of prostheses have been argued for a number of
reasons. The varying reasons help to highlight some of the issues that
stakeholders consider need to be addressed through the current reform process.
The key reasons and the arguments supporting them have been included below.
4.14
The main argument put by submitters to this inquiry is that the
definition of prostheses should include medical devices that do not fit the
current definition of prostheses but which provide superior outcomes. Cochlear
Limited submitted:
A revision of the definition of 'prostheses' and the
introduction of a benefit re-evaluation process as part of the Prostheses List
framework is recommended as way of ensuring the most cost effective
interventions are provided and appropriate reimbursement benefits are
maintained.[11]
4.15
Dr Janet Wale, formerly a consumer representative on the PLAC, states
that there is inequitable access to medical devices for privately insured
patients in private hospitals, in part due to how prostheses are defined for
inclusion on the PL. She argues that, 'It is time that we work to develop
consensus on an easily understood, publicly defensible definition.'[12]
4.16
In her submission, Dr Wale provides a list of medical devices, for
example, cardiac ablation catheters, which, despite having an evidence base for
their use, are unavailable in private hospital settings as they cannot be
included on the PL.[13]This
view is supported by many other stakeholders.[14]
4.17
Device manufacturer, Applied Medical, argues that the current definition
of prostheses goes beyond the original intention of the PL, and now includes
'low value surgical consumables which clearly do not meet the criteria
considered by Parliament when the regulation of prostheses was first adopted.'[15]
4.18
Applied Medical argue that the definition of prostheses be reviewed, but
with a view to rationalising the PL. Applied Medical argues that expanding the
PL to include other items ultimately will not work, because:
there will always be boundaries to what is, and is not,
included on the Prostheses List, regardless of which definition of what
constitutes a 'prosthesis' is selected. The inherent nature of the regulatory
framework is such that there will always be distortions in patient and
physician choice as a result of a misalignment of financial costs.[16]
4.19
Similarly, medical device sponsor, Biotronik, argues that items on the
PL, for example, coronary stents, may be used in preference to items that are
not eligible for listing, for example, drug-eluting balloon therapies, due to
the 'impact on provider revenues.'[17]
4.20
The committee has also heard that amending the definition of prostheses
may help in addressing one of the most pressing issues raised by stakeholders
during the course of this inquiry. LifeHealthCare states that:
incentives are being used by private hospitals to fund
prostheses that are not currently included on the PL (e.g. cardiac ablation
catheters or fractional flow reserve) due to limitations in PL definitions. We
believe this should be addressed by expanding the definition of a prosthesis to
include non implantable devices rather than relying on incentives, that are not
transparent, in order to gain access to these prostheses.[18]
4.21
This view is shared by another sponsor, Cochlear Limited. Cochlear has
suggested that, in addition to redefining prostheses, the role of the Health
Economic Sub Committee (HESC) of the PLAC should be expanded so that it can,
among other things, provide 'evaluation of cost effective interventions that
utilise medical devices that fall outside the current definition of
"prostheses"'.[19]
4.22
One submission has suggested expanding the definition of prostheses
through the creation of a 'Part D' for the PL, to enable inclusion of medical
devices which cost over $300 and which are single use but are not implanted.[20]
4.23
The IWG noted in its report that representatives from the private health
insurance sector argued that consideration of a review of the definition of
prostheses 'must only occur in the context of a revision of the benefit
setting.'[21]
What should legitimately be
included in setting a benefit level?
4.24
Considerable debate has occurred during this inquiry in relation to
whether the benefit level for the reimbursement of prostheses should include
support services associated with a prosthesis.
4.25
Mr Chris Rex, Chief Executive Officer, Ramsay Health Care Limited,
questioned the current system thus: 'it has always intrigued me—and continues
to do so—why prostheses are actually treated separately from all other cost
inputs into procedures.'[22]
4.26
The department has advised that, currently, applications to list a new
device on the PL are required to propose a benefit for the device which would
cover the costs of both producing and supplying the device to the patient. In
some cases, ancillary services and after care are also included in the benefit.[23]
4.27
Biotronik Australia Pty Ltd, which manufactures and supplies cardiac and
combined drug and device therapies, notes that in relation to the current
system:
the PL benefits were negotiated on the basis that the benefit
should include all costs associated with delivery, implantation and support of
the prostheses and hence the publicised significant benefit differences between
public and private are justified.[24]
4.28
Biotronik argues that there are sound reasons why services are included
in the costing of prostheses for the PL, citing as an example where these
services are included in professional bodies' standards of care for patients,
such as those set by the Cardiac Society of Australia and New Zealand.
Biotronik states that in public hospital settings these services are usually
provided through clinics in the hospital, but in the private sector, are often
delivered by surgeons with the assistance of the medical device sponsors.[25]
4.29
Other medical device sponsors, for example, Applied Medical, state that
it provide the same type and level of service in relation to their PL devices
regardless of which setting is used, public or private hospital.[26]
4.30
Cochlear Limited also submitted that it provides the same level of
professional training, and patient management, and support in both public and
private hospital settings, and listed the annual cost of doing so in its
submission.[27]
4.31
However, Cochlear, which does not have high a volume of sales, supplies
prostheses to public and private hospitals under the same purchasing
arrangements, that is, on individual orders per patient.
4.32
Cochlear argues that product management and support of devices on the PL
should be factored in to the benefit levels set.[28]
4.33
AusBiotech, a network of Australian small and medium companies in the
life sciences industry, including medical technology, argues that any
evaluation of and changes to benefit setting methodologies should consider that
the:
Totality of the service delivered is taken into account when
determining the value of the prosthesis benefit for a product, i.e., not only
'clinically relevant requirements', as mentioned by the IWG, but also other
aspects of service (e.g., education provided), the relative size of the private
market, attractiveness of Australia for launching new technologies, etc.[29]
4.34
The committee notes that this appears particularly relevant for the
small to medium Australian medical device sponsors, which may supply prostheses
to both sectors without significant variation in price, and which may be most
impacted by significant changes to the current benefit setting framework.
4.35
In its evidence, the private hospital operator, Healthscope Ltd, stated
that it negotiates contracts with medical device sponsors which enable the cost
of handling of prostheses to be covered through those contracts.[30]
4.36
Healthscope Ltd also indicated that prior to the current PL framework:
there used to be a handling fee of five per cent applied to
the value of a prosthetic device that was designed to compensate the hospital
for the cost we incur in moving devices in and out of the hospital and the
like. From a Healthscope perspective, if the outcome from this review was more
transparency on pricing and some form of handling fee in that range that was
designed to compensate hospitals for costs incurred, that would be an outcome
that we think we would not be uncomfortable with.[31]
Committee view
4.37
The committee notes that the majority of stakeholders have supported a
review of the definition of prostheses and reconsideration of what should be
included in setting a benefit level for prostheses reimbursement. The committee
also notes that the PLAC has included a review of the listing criteria,
including revising the definition of prostheses and longer term work on
revising how benefit levels are set, in its work plan.
4.38
In undertaking a review of the definition of prostheses and other
fundamental changes to the current system, consideration should be given to the
range of issues raised by stakeholders, through this inquiry and other earlier
reviews.
4.39
The committee notes that several submitters commented on the use of
medical devices that are not surgically implanted, or that do not require
hospitalisation to implant.[32]
There is a need for consideration of how these kinds of devices can be
reimbursed.
4.40
The committee considers that opportunities to redefine the criteria
should not be limited to expanding the definition to broaden the kind of
devices included.
4.41
In particular, it is important for the PLAC and the department be
mindful of, and take steps to address, the broader implications of changing the
definition of prostheses, within the context of other proposed reforms.
Consideration should also be given to how changes to the definition and listing
criteria may address some of the ongoing issues raised by submitters, including
finding a more transparent and robust way of setting benefit levels.
Setting a PL benefit level – which model?
4.42
In addition to reviewing the definition of prostheses and the criteria
for listing, government reforms also include consideration of alternative
models for the setting of benefits for items on the PL.
4.43
The IWG was presented with some potential options, including
international reference pricing, price benchmarking using weighted averaging of
public and private sector prices, and the price disclosure model used for the
Pharmaceutical Benefits Scheme (PBS). Upon consideration of the various models
presented to it, the IWG agreed, in relation to possible models for benefit
setting, that:
Should Government seek medium term benefit reductions across
the PL, it would be appropriate to consider legislating a price disclosure
system for prostheses, encompassing both public sector and private sector
medical device pricing; [and] ...reference pricing taking into account domestic
and relevant international prices, be considered as a mechanism to set the PL
benefit.[33]
4.44
Work is now under way to research and identify possible models for
consideration by the PLAC, as outlined in Chapter 3 of this report. A
consultant has been engaged to research and compare:
-
pricing models, including reference pricing and market based
approaches;
-
international and domestic benchmarking;
-
price disclosure; and
-
benefit setting frameworks.[34]
4.45
A report by the consultant, providing a comparative analysis of benefit
setting models, was released by the department on 4 May 2017. The report looks
at international and domestic examples of benefit setting models and price
disclosure that will assist the PLAC in its work.[35]
4.46
The committee notes that there is also concurrent work being undertaken
by the PLAC itself, through consideration of applications to list novel devices
on the PL:
There are one or two [PL applications for novel devices] that
are in train where we are using it very much as the new frontier, if you like.
They are new and they do not clearly fit into any of the existing groupings,
and that is where I am working with my colleagues in MSAC [Medical Services
Advisory Committee] and the TGA [Therapeutic Goods Administration], because I
think what we need to do there is to do a proper evaluation, and in some cases
that has already been done by MSAC as part of their work for deciding on MBS
item numbers et cetera, coming up with a cost-effective price.[36]
4.47
The department has stated that for new prostheses to be used within
newly devised medical processes, the PLAC will take advice from the MSAC on the
cost effective price of the device in the context of the whole healthcare
service.[37]
4.48
The committee notes that the desired outcome of current work being
undertaken by the PLAC is that '[b]enefits on the Prostheses List reflect the
appropriate reimbursement costs of supplying medical devices to patients.'[38] In relation
to adopting new benefit setting models, there appear to be a wide variety of
options even within a particular type of pricing model, and possible
combinations of models that can be used.
Reference pricing
4.49
The IWG and the PLAC have indicated that consideration of reference
pricing, with possible domestic and international benchmarking, is being
undertaken to identify a possible longer term benefit setting model.
4.50
In effect, reference pricing would include comparing the price of
prostheses in the private sector in Australia with other markets, potentially
domestically (in the public health sector), or internationally and setting a
benefit level that was in line with comparable benchmarks.
4.51
There has been a mixed response to this suggested model. Some
stakeholders, notably medical device sponsors, have been critical of this
model, outlining the challenges of finding suitable comparators, either
domestically or internationally.[39]
4.52
Some medical device sponsors have indicated that it could usefully be used
as an input to a decision on benefit levels, but should not be the only
consideration, and would necessitate alternative approaches for new devices
where there is no comparator. In its submission, Cochlear Limited stated that:
International reference pricing (IRP) may be used as an input
into the benefit setting process for new technologies that have not yet been
launched in Australia. However, due to the complexity of adjusting for
differences in health care systems across multiple jurisdictions, there may be
significant challenges in defining algorithms to adjust median IRPs to inform
benefit levels of prostheses in the Australian Private Health Insurance
environment.[40]
4.53
Healthscope Limited supports the introduction of reference pricing
'while having regard to the different market dynamics in other countries.'[41]
4.54
Private health insurers consider reference pricing to be a suitable
option that has been successful in other countries and could be applied in
Australia:
Reference pricing is a well-accepted system which is
currently used in several countries. For instance, Japan has employed
international reference pricing for over a decade. France, Italy, the Czech
Republic, Russia and the U.K. are other exemplars of domestic or international
reference pricing. In applying this model to prostheses pricing in the
Australian health system, the proposed reform would closely resemble similar
recent reforms to the Pharmaceutical Benefits Scheme (PBS) where more stringent
requirements on price disclosure and international references are expected to
yield $3.1 billion in savings by 2018.[42]
4.55
Some medical device sponsors have recommended that any new benefit
setting model that relies on comparisons between different markets, in
particular the domestic public and private hospital sectors, should use
information at a billing code level.[43]
This would in effect mean that each individual medical device would be compared
to itself.
4.56
However, the committee notes that this issue was dealt with as part of
the 2009 Review of Health Technology Assessment in Australia, which found that
this approach adds unnecessary administrative burden and recommended that
groupings of devices be used as the basis for any benefit setting.[44]
4.57
As referred to in the PHA submission above, the current reform process
being undertaken by PLAC includes consideration of the model developed for the
PBS, which is also administered by the department.
Price Disclosure – the
Pharmaceutical Benefits Scheme pricing model
4.58
It has been suggested that the reforms that have been undertaken to
address issues with the pricing and reimbursement of pharmaceuticals in
Australia might also be applied in reforming the PL framework. The
representative body for not-for-profit private health insurers, hirmaa, sees
merit in applying a similar model of price disclosure to the PL:
We look to the PBS system as having some good examples of
what we could do in this space. We do not see a big difference between
pharmaceuticals and devices. We think there should be mandatory legislated
price disclosure consistent with the PBS or maybe even stronger, with very
harsh penalties for not disclosing.[45]
4.59
Some caution has been expressed by other stakeholders, including CHA, in
adopting a model based on the reimbursement of medicines, given the inherent
differences between medicines and medical devices:
I think there are challenges with that. Drugs—legal
drugs—take a long time to come to market. They have a patented molecule, and
generally that molecule will remain unchanged for years and years. If you look
at prosthetics—let us take a surgical stapling device—it is a little bit like a
car: each year there will be slight modifications to it, whether they are true
enhancements or not, the model number will change, the name will change and
there will always be this challenge of, 'Is it the same circular stapler that
was listed two years ago?'[46]
4.60
AusBiotech has similarly urged caution, and highlights the cost of
administrative costs to sponsors, particularly small to medium companies:
This [PBS] model has the potential to
improve price transparency and reduce rebates for existing categories of
products. However, the implementation and administration of this system
would be expensive and our members have expressed concern that the burden of
these costs would be passed on to industry.[47]
4.61
Applied Medical states that although price disclosure might be suitable
as part of a range of reforms, on its own it would not 'deliver the price
reductions necessary to see Australians paying market based competitive rates
for prostheses in the private health system.'[48]
Committee view
4.62
The committee understands that there are potentially several models that
may be used in the Australian context to help maximise cost effectiveness and
access to prostheses for privately insured patients.
4.63
It is clear from the evidence presented to this inquiry that
stakeholders are very aware of the options currently under consideration but
there are different views on the effectiveness of the various options.
4.64
The committee acknowledges that stakeholders have made significant
efforts to critique the various options and considers it important for any
further research and analysis to include consideration of this work in arriving
at final options for the government to consider.
4.65
It should be noted, however, that while no one model will suit all
stakeholders, there may need to be compromise and negotiation to ensure that
the final model is workable and delivers desired outcomes.
Whole of episode payment
reimbursement
4.66
Some submissions have signalled a desire to move eventually to a whole
of episode reimbursement model.[49]
For example, PHA stated that a:
longer-term opportunity is to integrate prostheses costs into
an episode-based payment. Agreeing on a predetermined reimbursement per
procedure (e.g. per MBS item) would create stronger incentives for
manufacturers to compete on price and improve the sustainability of the overall
health system.[50]
4.67
The MTAA does not support a move towards using payments which would set
a reimbursement level for the whole 'episode of care'. The MTAA states that
using such a model:
encourages the pursuit of the lowest cost product as opposed
to the highest value product (in terms of the contribution to the patient’s
health outcome). This will reduce the number of innovative products available in
the private sector and eventually erode the value of private insurance to
consumers, which, based on a Government survey from 2015 was already a
significant concern.[51]
4.68
Catholic Health Care indicated that benefit levels based on episodes of
care are likely to be the future, but there is a long way to go:
Inevitably, I think, the health industry should move to
episodic, value based purchasing. The challenges are that, in the private
sector, the vast majority of doctors are not employed by and are remunerated
separately from the hospital. Often their incentives are quite different to
those for the hospitals. That is why it really would require a restructure of
both the Medicare system and the health insurance system.[52]
Committee view
4.69
The committee acknowledges the extensive work undertaken by stakeholders
in considering the best approach in reforming the PL and in arriving at a
better way of considering the appropriate reimbursement level for prostheses
paid for through private health insurance.
4.70
It is clear that there are a number of options that might be suitable,
and choosing the right model should be a process undertaken with consideration
of the research, analysis and comparison that has been undertaken by
stakeholders, in addition to that being undertaken by Professor Clarke on
behalf of the PLAC.
4.71
Whichever method is chosen for the determination of benefit levels to be
paid by private health insurers, or whether the government ultimately chooses
to let the market decide what prices are paid by private health insurers for
medical devices, it is hoped that the Department of Health and the various
committees genuinely work together in a more coordinated way, and with
effective consultation to ensure the best possible outcome of the current
reform process, and ultimately, a better deal for taxpayers and consumers.
4.72
It is clear from the submissions and other evidence received by the
committee that there are many inter-related issues for consideration through
the reform process currently under way. There are significant problems with the
current model for the reimbursement of prostheses through the PL, many of which
have been raised on more than one occasion through reviews and also through the
research and analysis undertaken by stakeholders themselves.
4.73
As Stryker Australia has noted in its submission:
It is not possible to reform any individual component of the
health system without consideration of flow on impacts to other stakeholders.
As such, any reform must involve extensive consultation and a strong
evidence-based approach. Ad hoc or reactive reform enacted under short term
political or media pressure is unlikely to achieve the overriding goal of
sustaining a high quality and affordable healthcare system.[53]
Data collection
Device registries
4.74
The committee heard that the development of device registries, such as
the Australian Orthopaedic Association National Joint Replacement Registry
(AOANJRR), have played an important role in improving outcomes of surgeries and
identifying devices which were not performing to the same standards as
comparable devices. For example, the AOA submitted that the AOANJRR had shown
that large-head metal-on-metal hip joint 'lookalike prostheses' were not
performing to the same standards as the original product.[54]
4.75
In its final report the IWG agreed that there should be a formalised
process of post-marketing review and considered the absence of this process to
be a major failure of the current PLAC arrangements. In particular, the IWG
noted that the PLAC has failed to delist devices where there was evidence from
current registries of devices not performing as well as comparable products.[55]
4.76
However, the IWG also noted that the PLAC is not currently resourced to
introduce a post-marketing review process. The IWG supported more coordination
between the PLAC, registries and the TGA to identify both superior and inferior
devices.[56]
4.77
Currently registries such as the AOANJRR are maintained and funded by the
applicable medical college.[57]
The department levies device manufacturers and passes this funding on to the
relevant college.[58]
The information provided to inform the registry is voluntary but the colleges
receive a high percentage of involvement as this is best practice amongst
contributors to the registry.[59]
4.78
The department noted that in the future such information could be
automatically captured by the e-health system, stating that:
These things will need to be built in the e-health system,
but there is a future pathway to every operation and every device being in a
system... and then being able to capture down the track whether there is
re-hospitalisation in respect of those issues and devices.[60]
Independent Hospital Pricing
Authority
4.79
The IHPA collects data from both public and private hospitals in two
separate collections through the National Hospital Cost Data Collection (NHCDC)
process. The NHCDC includes information on costs associated with prostheses and
other medical devices.[61]
4.80
The data provided by states and territories on public hospitals is used
to determine the National Efficient Price which provides a price signal or
benchmark for the efficient cost of providing public hospital services. The
public sector NHCDC captures 90 per cent of acute admitted hospital activity.[62]
4.81
Information from private hospitals is provided to IHPA on a voluntary
and confidential basis and accounts for approximately 60 per cent of total
overnight private hospital separations.[63]
4.82
To ensure consistency of data provided by public hospitals, the IHPA
conducts quality assurance and validation processes, including an independent financial
review.[64]
However, data provided by private hospitals is not subjected to such processes.[65]
4.83
As outlined in Chapter 2, there is a significant difference between the
prices paid for prostheses in public and private hospitals and there is a lack
of transparency around the rebates and discounts received by private hospitals.
4.84
In answers to questions on notice, the IHPA provided information on the
costs of prostheses provided in private hospitals and noted a number of caveats
around comparison of data between public and private hospitals.[66]
In particular, the committee notes that there is a year's gap between the
information available on public and private hospitals, that private hospital
data is not subject to independent financial review and that the private
hospital data is based on an older version of the Australian Refined Diagnosis
Related Groups.[67]
Committee view
4.85
The committee believes that, in the absence of a formalised
post-marketing review by the PLAC, device registries play a useful role in identifying
prostheses which are not performing to an acceptable standard.
4.86
The committee supports better coordination between the PLAC, device
registries and the TGA to identify both superior and inferior devices and to
consider delisting poor performing devices from the PL.
4.87
The committee notes that the e-health system may have the capacity to
play the role of a single device registry in the future and that this would
enable the automatic collection of information on devices.
4.88
The committee notes that there are a number of factors which contribute
to a lack of transparency around prostheses pricing. Notably, information from
private hospitals is provided on a voluntary and confidential basis and there
are a number of caveats attached to private hospital's data which limit the
ability to compare public and private hospital data.
NDIS transition arrangements
4.89
The terms of reference for this inquiry include consideration of any
implications for prostheses recipients of the National Disability Insurance
Scheme (NDIS) transition period. The NDIS enables people with a disability to
access support through individualised plans.
4.90
The Department of Health submitted that 'it is unlikely that the
transition period of the NDIS will have any significant implications for
recipients of prostheses funded by the prostheses listing arrangements.'[68]
4.91
However, one submission to this inquiry did raise some concerns in
relation to the transition of Australian Hearing clients to the NDIS. Cochlear
Limited indicated that there is:
lack of clarity regarding the transition of Australian
Hearing (AH) clients to the National Disability Insurance Scheme (NDIS). This
is resulting in confusion and uncertainty individuals, providers and suppliers.[69]
4.92
The Cochlear submission states that '[n]o solution has been defined for
the ongoing support of some of Australia's most severely hearing impaired and
financially vulnerable - those AH clients > 65 years.'[70]
Navigation: Previous Page | Contents | Next Page