Clinical trials for people with low survival rate cancers
3.1
This chapter examines two distinct issues with respect to clinical
trials for people with low survival rate (LSR) cancers: barriers to accessing
trials and jurisdictional issues for trials.
3.2
The Garvan Institute of Medical Research/The Kinghorn Cancer Centre/The
Garvan Research Foundation (Garvan Institute) outlined in its submission the existing
ways in which patients without any standard treatment options can access
clinical trials, and noted that '[t]he first step in improving the outcomes for
rare and high-mortality cancers is to engage patients in the research
enterprise. Without data, nothing can improve'.[1]
The treatment access options are as follows:
-
phase 1 clinical trials – as these
are primarily focused on defining the toxicity profile of a new treatment, it
takes a long time to get sufficient numbers of participants and they are costly
and intensive, limiting the number of phase 1 studies that a single institution
can open at one time.
-
phase 2 or 3 clinical trials,
however, cost limits the number of phase 2 studies that can be run
simultaneously at any one institution.
-
compassionate access to new drugs
and off‐label treatment. This is common practice in Australia,
and while it may produce anecdotal insight into novel therapeutic
possibilities, these results are idiosyncratic, ad hoc, unsupervised and
unregulated, and mostly go unreported, thus failing to contribute to the body
of knowledge. Most importantly, ineffective treatment is likely to be
underreported.[2]
3.3
The Garvan Institute further noted that the 'two key barriers to
improved outcomes for less common cancers' are lack of access to clinical
trials, and lack of access to the best available treatments, which are
'[i]nextricably linked', because:
As governments use information gained from trials when
deciding if they will fund a new drug, it is critical that patients with less
common cancers have access to clinical trials, and that government, academics,
clinicians and the pharmaceutical industry work together to develop trials for
these cancers, as well as the more common cancers. Currently, there is a real
disconnect between the identification of a new treatment by researchers and,
where relevant, access to these treatment options.[3]
3.4
The relationship between clinical trials and the Therapeutic Goods
Administration (TGA), the Pharmaceutical Benefits Advisory Committee (PBAC) and
the Pharmaceutical Benefits Scheme (PBS), as well as philanthropic and
pharmaceutical funding for clinical trials, were examined more generally in
chapter 2.
Barriers to accessing trials
3.5
There are a number of barriers to accessing trials, including the
absence of trials for LSR cancers, identifying the availability of trials, meeting
the trial criteria and having the physical and financial means to participate
in a domestic or international trial.
3.6
The Walter and Eliza Hall Institute of Medical Research (Walter and
Eliza Hall Institute) identified the following 'recurring themes' with respect
to the challenges to establishing clinical trials in Australia:
- access is limited for patients with rare cancers, as
trials will not be available in all major treatment centres;
- access for patients in rural Australia is difficult when
the trial requires frequent attendance at a capital city centre;
- the time taken to establish a trial is
disproportionately long compared to the survival time of patients with low
survival cancer; and
- pharmaceutical companies are risk adverse when it comes
to initiating adequately sized trials in cancers with low incidence.[4]
3.7
Mr Tim Eliot identified several barriers to his participation in
clinical trials which caused him to accept standard of care treatments[5]
for his glioblastoma:
...admin did not provide details of the trial; existing
treatment timing meant the trial start date was missed by a week; my tumour was
in the wrong location; the trial was already full; the trials were not being
run in Western Australia; etc, etc.[6]
3.8
Mr Eliot opined that:
...these symptoms show the
current funding model is based on standard clinical research practices with a
limited number of patients able to be involved, and little, if any, data
sharing between trials.[7]
3.9
Mr Eliot argued that '[t]his approach is simply not working'[8]
and although he acknowledged that '[t]here are valid reasons for clinical
standards to be set high, particularly in researching new treatments',[9]
he submitted that 'standard, slow, phased clinical trials are not the only way
forward' and discussed the GBM AGILE model as an alternative.[10]
The Cure Brain Cancer Foundation (CBCF) noted that this particular trial had an
'innovative trial design' and 'an adaptive trial platform, which has great
potential to reduce timeline[s] through seamless transition from Phase 2 to
Phase 3 within the trial'.[11]
Access to this trial is further discussed in chapter 5.
3.10
The following sections examine some barriers to accessing trials that were
repeatedly cited during the course of the committee's inquiry.
"Dr Google"
3.11
In addition to people independently searching the internet for
information about inexplicable symptoms[12]
or about a disease following diagnosis,[13]
the committee heard that people resort to "Dr Google" to find
out information about access to trials. For example, Ms Marilyn Nelson, who has
lung cancer, described how she conducted her own research on clinical trials in
order to find 'some hope':
We are looking for news about trials and new drugs that are
coming along. There is not that much information about it here in Australia, so
we look to Google and we look to proper websites over there to try to find—just
some hope, you know? That is what we are looking for, that there might be
something.[14]
3.12
Professor Mark Hertzberg of the Australasian Leukaemia and Lymphoma
Group (ALLG) considered that it is now easier, with the internet, to access
information about clinical trials, noting that patients:
...come with wads of paper, particularly the relatives of the
patient and the children of the patient. The clinicians also have better access
[to information about trials] than ever before.[15]
3.13
Indeed, Professor David Walker opined that the approach of searching the
internet for treatments occurs '[a]ll the time', as:
...a patient with a brain cancer will be told they need an
operation, they will be told the diagnosis and 'This is what is going to happen
to you'. There is very little information given to them up-front and there is
certainly no information in almost all circumstances about available trials and
what that might have to offer.[16]
3.14
Ms Julie Marker of Cancer Voices Australia also suggested that
clinicians may be unfamiliar with LSR cancers and associated trials, which in
turn can raise a whole host of problems:
Often clinicians are not so familiar with these rare cancer
types and the trials. So there may well be opportunities for treatments that
people are just not aware of both from the clinicians and from the consumers
side of it to find the best treatments, or even the clinicians who are in any
way familiar with treating these conditions. Often that means travelling to
other locations. Again, you have to be wealthy enough to afford to do that
because that is not supported. There is the potential for duplication if there
is not some register of even the preliminary pilot studies.[17]
3.15
This issue about the lack of information available to patients was also
reflected in the evidence from Mr Evan Shonk and Mrs Suzanne Turpie:
CHAIR: One of the other things that a number of you
have mentioned was clinical trials. I am interested in what sort of information
you were given about clinical trials and how much you had to go away and
research for yourselves.
Mr Shonk: There is virtually none available. They
pretty much do not exist.
CHAIR: Someone mentioned to me that there are not even
any brochures available about brain cancer.
Mrs Turpie: Yes. We did not encounter any brochures.
We were just sat in a room and told, 'This is a clinical trial that kids with
medulloblastoma are on.' To be honest, there was nothing given to us, it was
scary and I felt like my son was being used for research himself while he was
still living.[18]
3.16
Similarly, in her evidence to the committee, Ms Jill Emberson, who has
ovarian cancer, expressed her surprise that 'there is not more clear
information available about running trials', but also identified why this may
be the case:
...I understand that all the people running the trials are so
strapped in even getting their trials up and running and that the
administrative support, as I understand, is also a real barrier to people
running the trials inside the hospitals and the labs. And that that would be stopping
trials getting up and running, I find, gobsmacking.[19]
3.17
Mr William Williams, whose wife passed away from a GBM grade 4, also spoke
of his experience of finding out information about trials:
There is a website that I did look at, which did not really
lead me anywhere in particular to the possibility of a trial. So it was in fact
drawing on the experience of Denis and other people I knew in the brain tumour
area, and just saying, 'Who do you think might be running a trial?' Denis said,
'Well, you can call so and so in Melbourne', and I did. After announcements of
other initiatives in cancer research in this country, I called and just said
who I was. But there was no coordination or leading me in any way that showed
me a direction where there could be a trial. So you just cold-call and say who
you are and say, 'Can you help?' because it was not available.[20]
3.18
As Mr Todd Harper of the Cancer Council Victoria (CCV) observed, the
motivation of cancer patients to seek out the clinical trials available to them
'speaks to the value of having information that is consumer friendly and is
able to guide them towards these types of activities'.[21]
AustralianClinicalTrials.gov.au
3.19
The committee was informed that information about trials is available on
the AustralianClinicalTrials.gov.au website, developed by Cancer Australia in
partnership with the Australian New Zealand Clinical Trials Registry, the
University of Sydney and Cancer Voices.[22]
3.20
Dr Alison Butt of Cancer Australia provided the following
information about the website:
...the Cancer Australia website supports the only national
cancer clinical trials website which gives consumers access to current clinical
trials in Australia and to Australian arms of international trials. A
particular focus of the website is that it's consumer friendly. So there are
consumer lay descriptions of the trials, which obviously help when patients are
looking for appropriate trials. There are simple search functions which enable
them to navigate through the site and find trials that are eligible. In
addition, there's also specific information about the eligibility of the trials
and the implications of the trial participation. So the focus of the website is
really aimed at trying to encourage participation by making it a very
user-friendly experience.
As you alluded to, the data for the Australian cancer
clinical trials website is sourced from the Australian New Zealand Clinical
Trials Registry, the ANZCTR, but also ClinicalTrials.gov, which is the US
clinical trials website. It is dependent on the clinical trials being
registered, and the responsibility rests with the investigator and sponsor of
the clinical trials, so that is potentially a challenge. It is their
responsibility to update and provide information on that website.[23]
3.21
Although this description indicates that the website contains a wealth
of information about clinical trials, the committee heard that people living
with cancer are not accessing this information due to the difficulty they
experience navigating the website.[24]
3.22
Mr Greg Mullins of Research Australia proffered why this may be the
case:
I think it is extremely difficult for individual patients to
know what clinical trials might be suited to them. In nearly all cases they are
going to be relying on their treating doctor to be able to assist them to
understand whether they are eligible or not. There are searches that can be
done and, if someone perhaps gets really lucky and really knows what they are
doing, they might be able to find that information, but most people are going
to be relying on their doctors to assist them with that.
We have undertaken public polling in the past, where we have
asked people about clinical trials: are they aware of them and what they are?
Typically, what they are telling us is: 'I rely on my doctor.' That is very
much where it is at. I know the last speakers were talking about the difficulty
of understanding even the range of clinical trials happening within Victoria.
On a global scale, that is enormous, and it is not the patients who are in a
position to do that. It really is a matter of ensuring that our researchers and
our organisations are connected globally and understand what is happening.[25]
3.23
Indeed, Professor R John Simes considered that doctors are the 'main
people' who view the AustralianClinicalTrials.gov.au website, but noted that the
website, which contains 'a lay description so that the information is in less
threatening terms', is also accessible to members of the community.[26]
Despite this, Professor Simes considered that improvements could be made to the
website, as:
...to work out whether a particular trial is suitable for a
particular patient still requires a discussion with their doctor et
cetera...while one thing is to be able to find out what trials are available, the
other thing is—if the trial is not available at your site, in your city or at
your hospital—how can you get access to other places. They are really important
issues.[27]
3.24
This was reflected in Mrs Madeline Bishop's submission, where she
asserted that, when looking at the website:
...one needs to know exactly what one is looking for to be able
to locate and be included in a trial. When looking for non-government or
partially funded trials, one must seek information from the individual groups
and their current trials. This haphazard method is not good enough for the
individual whose health and wellbeing is already compromised by their cancer.[28]
3.25
Indeed, Ms Susan Pitt also informed the committee that some trials, such
as physician-led trials, may not be listed on the website.[29]
3.26
In its submission, the National Health and Medical Research Council (NHMRC)
referred to survey results which 'demonstrate that the lack of awareness of
relevant trials is a barrier, not just to increased participation, but also to
increased cross-referral of patients by general practitioners or clinicians'.[30]
3.27
In response to this, the NHMRC has been 'working to improve recruitment
into and awareness of clinical trials' in the following ways:
- enhancing the functionality of the
AustralianClinicalTrials.gov.au website to bring together resources for
consumers, participants, researchers and proponents of clinical trials, and as
a tool to encourage patient recruitment, and
- developing a national marketing campaign to improve
awareness of the website and an understanding of the role and value of clinical
trials. Funding for the campaign has been provided by the Department of
Industry, Innovation and Science.
3.28
The NHMRC noted that '[i]mprovements in cross-referral rates of GPs and
clinicians have also been observed through the use of a Mobile Applications
(‘Apps’) - ClinTrials refer'.[31]
3.29
However, in response to a question about the accessibility of the
website, Adjunct Associate Professor Christine Giles of Cancer Australia conceded:
We can certainly look at different ways of directing people
to the website—through social media and some of our existing mechanisms. The
consumer organisations, we would anticipate, would do that as well. But, given
the comments that you're making, we would certainly be able to have a look at
that.[32]
3.30
Mr Harper spoke to the committee about the CCV's clinical trials website,
Victorian Cancer Trials Link (VCTL), informing the committee that, following a
redevelopment, the CCV had successfully made the website 'more user-friendly
and searchable for individuals' and as a result, 'there has been quite a lot of
interest right across Australia' and internationally.[33]
Mr Harper elaborated on the redevelopment process:
The recent website redevelopment was done with patients. We
wanted to make sure that the final product was one that was very user friendly.
Since the redevelopment of the website we saw in May this year the website
attracted 3,130 visits from users, which was a 30 per cent increase from prior
to the introduction of the new website. At least on those initial numbers we
are very confident that it has responded to the need of cancer patients.[34]
3.31
Mr Harper suggested that the CCV clinical trials could be 'made
available more broadly', noting that:
I am sure that my Cancer Council colleagues, for whom
clinical trials is a priority, would be very happy to work on expanding what
was essentially a prototype developed in Victoria and making that available
nationally. Obviously, having a site that is already established and has
demonstrated feasibility may offer some advantages.[35]
3.32
However, Mr Harper identified that some issues would need to be
addressed, including:
...encouraging clinical trial sites to contribute data. That is
done under a funding model in Victoria, as I said. Currently it is about
$200,000 in Victoria. Ideally, we would like to increase that in Victoria to
make that available or provide a greater incentive for organisations to submit
their trial's information. I do not see any reason why that could not be looked
at as a prototype that could be rolled out across Australia. My guess is that,
if that was done, funding would be between $1 million and $2 million, probably
closer to two, to enable incentives for trial sites to submit their data and
also to upkeep the website and promote that website.[36]
3.33
Mr Harper outlined the benefits of this approach:
The two principal benefits of that that, I see, are: firstly,
to provide access in a form that has been demonstrated to work well with
consumers; and secondly, to enable trial sites to use that to recruit patients
to their clinical trials. I think that that would be quite a substantial
benefit as well. I should also note that the Ian Potter Foundation was very
generous in providing us funding to enable the website to be recently
redeveloped.[37]
Eligibility for trials
3.34
Many people who have LSR cancers may be ineligible for trials because of
their current state of health, prior treatment, or their age.
3.35
For example, Ms Linda Ferguson, who lost her wife to brain cancer,
informed the committee that:
We asked our various specialists in Canberra and in Gosford
if there were any trials that were suitable. We were told that there were not.
We did research online to see if we could come up with anything, but we found
that, once you make particular treatment choices, you are given a particular
drug or the tumour recurs, suddenly anything that might have been eligible you
are no longer eligible for, because you have already had another drug. So the
doors close very quickly once you have made treatment options. With time being
such a pressure, you make those treatment decisions as quickly as you can.[38]
3.36
Mrs Raechel Burgett, who has a grade-3 oligoastrocytoma, stated that to
access certain trials, she would need to be on her 'deathbed':
I looked and I applied but it is all for grade 4 astrocytomas
because that is the worst and the deadliest. They are opening all the trials
for them, and even at that stage it is not until you are terminal that they
really let you in. I am someone who is still relatively early in their
diagnosis and who has a few years up their sleeve, and so they will not me let
in until I am on my deathbed.[39]
3.37
Mrs Tracy Taylor also described how her son, who has brain cancer, could
not access trials for various reasons, including his prior treatment and age:
My son has already had the gold standard of treatment and
radiation a couple of times, so that in itself makes him not applicable for
trials. His age as well makes him not eligible for trials. If they know from
the start and they have other treatments, as they are calling them, or ones
that are yet to be made and they are yet to trial that they can maybe go on
this path of this new thing, as opposed to just doing the gold standard of
treatment, which then makes them not eligible to do other trials.[40]
3.38
The timeframe within which a person can be eligible to begin a trial can
also be quite tenuous. For example, Ms Simone Leyden of the Unicorn Foundation recounted
a story of a patient who managed to join to a trial after her oncologist initially
informed this patient that no trials were available. Ms Leyden noted that '[i]f
she had literally waited another 24 hours, she would not have been eligible for
that trial'.[41]
3.39
In addition to the eligibility criteria, Ms Nelson informed the
committee that '[t]here is a strict protocol' when you are in a trial,
explaining that:
I cannot have had this and I cannot have had that to get into
the trial. Then, while I am on the trial, I cannot use any other therapy. If I
do, my doctor would have to agree to it. The only reason they would stop the
trial would be if the trial ends or I get progression, which is going to be
picked up on one of the regular scans and then I am bumped out of the trial and
we find out which drug I was actually on. That then decides what is
next—whether it is chemo next or whether there is actually another targeted
therapy that I can try. Yes, there are very strict guidelines for getting in
and there are certainly very strict guidelines—you cannot undertake any other
treatments while you are on the trial. But it is better than the alternative.[42]
3.40
In its submission, the NHMRC noted that the criteria for eligibility 'are
usually determined by the clinical trial sponsor', such as a pharmaceutical
company or a clinical trial network.[43]
It was noted that:
Paradoxically, a sponsor’s legitimate aim to reduce
confounding factors and thus ensure that a clinical trial produces the highest
quality evidence of efficacy, may result in narrow eligibility criteria that
significantly lower recruitment.[44]
3.41
Dr Melissa Grady of AstraZeneca explained why inclusion/exclusion
criteria are in place:
It is not an exclusion by want of exclusion. It is simply
that, if you follow the science and you want to make sure you have answered
that scientific question of that drug or innovative therapy, you must be quite
rigorous around the protocol that you design. By virtue of that, it means you
have a certain population to study and study very well so that you get the
right answer at the right time and that you are not wasting time as well.[45]
3.42
On the other hand, the eligibility criterion used by Professor David Thomas
of the Garvan Institute for his work in advanced genomics and personalised
therapy for people living with incurable rare cancer, is that his patients are
unable to access other trials:
...we have actually designed our modules with exclusion
criteria that say: 'The diseases where there are existing trials which people
can get access to are excluded from this because there are other trials
available. It is the people who do not have the trials available that we are
selectively screening.' And we have 170 of those within nine months; we have
600 by the end of this year. There is a huge population that just cannot get
access to trials.[46]
Australians in regional and remote
areas
3.43
People with cancer in rural and regional Australia also face additional
barriers in accessing clinical trials. This was illustrated in the submission
received from Mr Denis Strangman AM, whose wife passed away 11 months
after her diagnosis with a glioblastoma multiforme grade iv brain tumour:
...as a general rule, patients from regional centres do miss
out, unless they can travel to a major centre. From my knowledge the Canberra
Cancer Centre, as an example of a regional centre, has not so far participated
in an adult brain tumour clinical trial locally, although some of its patients
have – by travelling interstate.[47]
3.44
Ovarian Cancer Australia observed that:
Patients from rural and regional areas opt out of trials
because of the long distances travelled, the cost of travel and finding
accommodation and the rigours of travelling while feeling unwell from their
illness or the treatment they are undergoing.[48]
3.45
The committee also heard from numerous witnesses about the variations in
survival for people living with cancer in regional and remote areas versus
those in metropolitan areas.[49]
3.46
For example, CCV noted that '65% of people with cancer living regionally
survived five years after diagnosis, compared to 69% of people living in a
metropolitan Melbourne region', and provided the following table which
illustrates the five year survival rate for metropolitan and regional
integrated cancer service regions in Victoria, with the regional integrated
cancer services highlighted.
Table 5: Five-year
survival rates for Victorian Integrated Cancer Services[50]
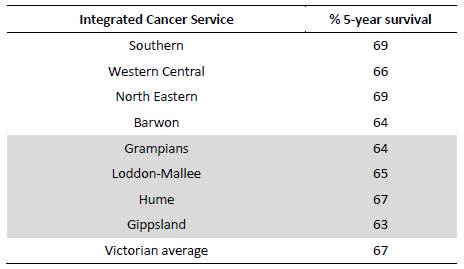
3.47
CCV also provided information about the differences in five-year
survival for low survival cancers between metropolitan Melbourne and the rest
of Victoria, which illustrates that, in many instances, people with LSR cancers
living in regional areas have poorer survival outcomes compared with those in
metropolitan areas.[51]
Table 6 presents this data over a five-year period.
Table 6: Five-year
survival for low survival cancers between metropolitan Melbourne and rest of
Victoria[52]
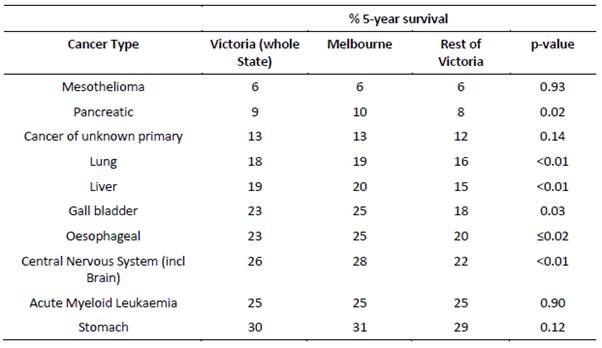
Funding for travel
3.48
In discussing the barriers to clinical trial participation, the
Victorian Comprehensive Cancer Centre (VCCC) submitted that, although the
'largest regional centres can conduct clinical trials, as they have the economy
of scale required':
...a recurring theme in recruiting for clinical trials is that
patients from rural and regional areas opt out of trials because of the long
distances travelled, the cost of travel and finding accommodation and the
rigours of travelling while feeling unwell from their illness or the treatment
they are undergoing.[53]
3.49
In his evidence to the committee, Associate Professor Gavin Wright of
the VCCC identified the 'regional-rural problem' as the 'No. 1' struggle in
recruiting people for clinical trials:
The kind of surgeon I am is not a common surgeon, so the
practice tends to come to me. I look after people from Launceston,
Albury-Wodonga, even Adelaide, Mount Gambier and all of Victoria. If I have a
trial on at my institution someone from Mildura or somewhere does not get any
reimbursement to turn up for a trial presentation. They can only get what
limited funding there is from state governments for assistance for actual
clinical presentations only, not for turning up to a trial test.[54]
3.50
Indeed, in advocating for centres of excellence for people with neuroendocrine
tumours (NET), Ms Leyden observed that:
The problem, obviously, is that most of those centres are
located in metro areas, and we see a huge burden for regional patients. We run
a NETs patient support line, which is just our nurse who works very hard three
or four days a week on the telephone, and we see that about 40 per cent of
those calls come from regional areas. So what we would foresee is, yes, those
patients still need to be actually funded or helped to go and be seen at these
centres of excellence...[55]
3.51
The ANZCHOG National Patient and Carer Advisory Group similarly observed
that '[w]here a trial is only available interstate, participation requires
funding for interstate travel and accommodation', which is 'a huge financial
burden for interstate patients', as currently, there is no funding available.[56]
3.52
In his evidence to the committee, Mr Dan Kent of the Australasian Gastro‑Intestinal
Trials Group, stated that in New South Wales, 'we get $60 a night to travel to
a treating centre, and that really does not cover too much. It would be nice if
those costs could be encompassed within trials to get regional, rural and
remote people in'.[57]
3.53
In contrast, patients participating in a pharmaceutical clinical trial
will generally be reimbursed for travel and other costs associated with
attending appointments, unless these patients are on a cooperative group or
investigator-initiated (that is, non-commercial) trial.[58]
3.54
In its submission, Ovarian Cancer Australia recommended 'expanding
medical travel and accommodation reimbursement schemes to include registered
clinical trial participation' in order to 'overcome the reluctance displayed by
some rural and regional patients who would otherwise be ideally suited to
participate in clinical trials'.[59]
3.55
The Cancer Council Australia (CCA) and Clinical Oncology Society of
Australia (COSA) identified the lack of financial assistance as a barrier to people
living with cancer participating in clinical trials and provided the following
information about existing subsidy schemes:
Financial assistance to support travel for specialist medical
services that are not available locally are offered by state and territory governments
and administered through public hospitals. Currently, patients who choose to
participate in a clinical trial do not qualify for these schemes. For the
patient, this can reduce their available treatment options and for the
researcher, it can limit representation of the rural and remote population in
their study.
The various patient travel subsidy schemes lack flexibility
to respond to complex circumstances of individual patients, constrain decision
making and segregate eligible patients from participating in clinical trials.
Additionally, these programs are under-funded and do not meet the real life
costs of travel and accommodation. The schemes do not ensure a patient has
equitable access to all treatment options regardless of geographic location, and
in the interests of the individual and the public, the Government must
encourage participation in clinical trials for all cancer patients regardless
of geographic location.[60]
3.56
Further, CCV provided the following figure which illustrates the
variation in reimbursement for patient transport assistance across Australia.
Figure 8: A comparison of
patient travel assistance schemes across Australia[61]
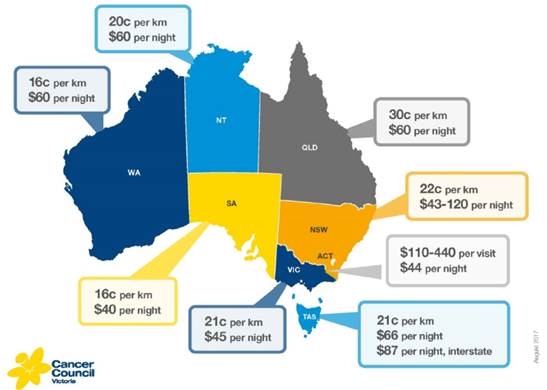
3.57
In order to respond to the barriers experienced by people with cancer in
regional Victoria, CCV, together with Cancer Trials Australia and the Victorian
Cancer Agency:
...have funded a three-year project to improve cancer patient
access to clinical trials conducted at regional centres. This is one of four
projects aiming to implement innovative solutions to increasing patient access
to cancer clinical trials. It is intended that the learning from these projects
will be applied to improve access to trials at other centres.[62]
The Australian Teletrial Model
3.58
Participation in teletrials is another way in which the barriers facing people
living with cancer in regional and remote areas may be ameliorated.
3.59
A 'teletrial' encourages the 'accrual of patients to a suitable clinical
trial regardless of geography within a state' by the use of technology to
reduce the need for patients to travel to institutions where the trial is
taking place.[63]
Mr Richard Vines of Rare Cancers Australia opined that '[t]eletrials are the
only way that people in the regions...are going to get access to state-of-the-art
treatment through clinical trials, if we can somehow build a protocol and
manage that remotely'.[64]
3.60
The Australian Teletrial Model, developed by the COSA Regional and Rural
Group, and endorsed by the COSA Council:
...outlines the key considerations for increasing access to
clinical trials for people with cancer living in rural and remote communities, and
facilitate study activity across rural and remote locations...[and] has the
potential to connect research centres, and improve the rate of recruitment to
highly specialised clinical trials, including low incidence cancers.[65]
3.61
The Walter and Eliza Hall Institute advocated for the support of the
Australasian Teletrial Model, which it submitted would 'encourage accrual of
patients to a suitable clinical trial regardless of geography within a state'.[66]
3.62
In their submission, the CCA and COSA provided the following information
about the model:
The model documents a feasible and effective tele-health
strategy to increase access to clinical trials closer to home using traditional
video-conferencing technology and web based systems. In addition, the model
will aid collaboration and networking between centres. This will have a flow on
effect for delivering greater engagement in research activity, improving
adherence to evidence based practice, improving the rate of recruitment of
patients into clinical trials, reducing the disparity in cancer outcomes for
geographically dispersed populations, building clinical trial capacity, and
providing trial-related training.
Since 2011, utilisation of tele-health in the delivery of
services has increased. In the first quarter of the 2011/2012 financial year
1,809 claims relating to telehealth services were processed through Medicare
compared to 40,570 in the quarter ending 30 June 2016.[67]
3.63
The CCA and COSA suggested the establishment of site specific governance
for accredited trial sites in public institutions, to be coordinated at a state
and territory or national level, and also supported the Australian Teletrial
Model, proposing that:
...an ‘accredited trial site cluster’ could be a network of
institutions identified as having clinical trials capacity as an established
multi-centre collaborative. The level of support provided to the smaller sites
would be determined by the complexity of the trial and the clinical
capabilities at the site. Increased capacity could be provided from the primary
site to potential rural and remote locations through tele-trial models and use
of e‑technology, such as the Australasian Tele-trial Model.[68]
3.64
To illustrate the way in which the Australian Teletrial Model could
operate, the Walter and Eliza Hall Institute, a founding partner of the model,
provided the following example:
...patients in Victoria would have access to a trial open in
Victoria at the closest comparable hospital. ‘Teleoncology’ models of care
offer the opportunity for patients living outside major metropolitan centres to
access clinical trials closer to home, reducing the need for travel...While the
principles of operation for primary and satellite centres are the same,
site-specific governance and processes need to be developed for effective implementation.[69]
3.65
Ovarian Cancer Australia also expressed its support for the model.[70]
International trials
3.66
As noted above, the rarity of LSR cancers means that there may not be
enough patients in Australia to conduct a stand-alone clinical trial. Indeed, Professor Walker
noted that:
...the barriers to running these trials is actually obtaining
numbers for rare cancers, and that is a common thing with all rare cancers. But
if you could get all the patients with brain cancer in one centre and available
for trials then I think that would accelerate improvements in outcomes. I think
that is the difference between here and Europe.[71]
3.67
The effect of the small number of patients with LSR cancers in Australia
on the ability to establish clinical trials was also reflected in the evidence
of Dr Chris Fraser of the Australian and New Zealand Children’s
Haematology‑Oncology Group (ANZCHOG), who spoke to the importance of
international partnerships in continuing to 'provide world's best care for
Australian children with cancer':
That is because our population is very small compared to that
of North America or the larger European countries. That means that we really
cannot run these clinical trials by ourselves in this patient population; we
have to be part of these international collaborative groups. The numbers for
each of those trials are becoming smaller and smaller as the subgroups that are
eligible for those trials get smaller. For example, for a particularly
molecularly targeted drug there is only going to be a small percentage of a
certain type of tumour that will be eligible for that trial. So international
cooperation and collaboration is increasingly important.[72]
3.68
Dr Fraser noted that he informs his patients about international trials,
because '[i]f we do not tell them, the age of the internet is such that they
find out about them very quickly':
It was probably five or six years ago that you could look
parents in the eye and say, 'There really is nothing else anywhere in the world
other than what we can do here.' That is not the case sitting here today. There
are treatments available overseas, some of which have very promising results
for very high-risk leukaemias that are proving to be very efficacious.[73]
3.69
However, Dr Fraser also informed the committee about the significant
cost of participating in international trials:
For me to send a patient to North America where they could
access one of these trials costs close to $500,000 to $700,000 for them to go
and enrol on that trial. That is something that parents now in Australia have
the knowledge about and have to deal with. I guess those cells are going to
come—they are in clinical trials. We need to position ourselves to be an
attractive enough partner that we can participate in those clinical trials, not
just in those cellular therapies but other new drugs. It is a rapidly moving
field. Our model, which has served us very well, has been to put our hand up to
be part of these trials and do it on the cost of the smell of an oily rag. And
that just does not work for these new trials. We need to work out a way that we
can continue to be attractive partners and continue to have early access in the
setting of clinical trials for these new and exciting drugs, so that parents do
not have to start looking overseas.[74]
3.70
Indeed, as Professor Terrance Johns identified, access to international
trials for Australians was not a regulatory problem, but a funding problem:
Prof. Johns:...Unless the company provides money to
specifically do an arm of the trial here or do the trial itself here, they just
will not run it. I try to work in that space a bit, but I think we can sell it
better. Internationally, I think we are very competitive, especially with the
dollar at 74c. I think it could be very attractive. Americans could do trials
here at half the price that they can in the US. I am also on the management
committee for COGNO, which is the major body that oversees clinical trials for
brain cancer in Australia. We have a very coordinated system across all states
in all the major teaching hospitals where we can run these trials; and we do
run them, but—
Senator SMITH: We could run more.
Prof. Johns: we could run more. We certainly have the
capacity to run more. It is trying to engage with industry in the US and Europe
to come and do some trials here, but we could do more. It is difficult. I
applied to do a trial through the new innovation grants, and it got knocked
back because they did not see enough value for Australia moving forward. So we
are trying to do that.[75]
3.71
The issue of funding was also reflected in Mr Dustin Perry's evidence to
the committee:
There have been times when [the oncologist] has told me that
there have been clinical trials running in other countries and they are happy
to enrol patients from Australia, but with an international clinical trial, if
the principal investigator for that trial is in another country, not in
Australia, you are instantly ineligible for government funding. Because a lot
of brain cancers, particularly paediatric ones, are so rare, there is not
enough of them in Australia to run a meaningful trial at all. The way the
funding system is set up literally discriminates against brain cancers and
others that are rare.[76]
3.72
Mrs Suzanne Turpie spoke to her frustrations with accessing domestic
clinical trials for her son who has brain cancer, when there are trials
available overseas:
We seem to have a standard treatment here depending on the
cancer and then an option of a clinical trial; however, if you look overseas,
there are options for treatment. Why are those options not available here? Why
are those drugs not available here? Why do we have people here in Australia
having to crowdfund huge amounts of money—in the hundreds of thousands of
dollars—to be able to go overseas to be given the opportunity to fight for their
child's survival? They talk to a doctor here and are told: 'There's nothing
more that can be done. Go home and wait for your child to die.' This is
heart-rending, this is real and this has been said.[77]
3.73
Following the presentation of the above evidence, on 24 August 2017,
the Australian government announced that it will co‑fund, together with
the Robert Connor Dawes Foundation, ANZCHOG's AIM BRAIN,[78]
'an international collaborative trial that will enable diagnostic molecular
profiling of children with brain cancer'.[79]
The duration of the AIM BRAIN is four years, and was accessible from 31 October
2017.[80]
3.74
As discussed in chapter 2, the government also announced on the same
date $13 million of funding for competitive research grants from the MRFF
'designed to boost clinical trial registry activity with priority given to
under-researched health priorities, such as rare cancers and rare diseases'.[81]
3.75
Further, as discussed in chapter 5, on 29 October 2017, the Australian
government announced the Australian Brain Cancer Mission, a $100 million fund
to defeat brain cancer.[82]
International comparisons
3.76
The following figure illustrates the number of total oncology trials
which started between 2007 to 2016, across Australia, China, the US, the United
Kingdom (UK), Canada and South Korea.
Figure 9: Phase
II/III and III oncology trials, by year of start-up - for China, USA, UK,
Canada, South Korea and Australia[83]
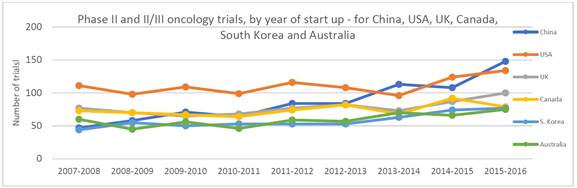
3.77
As this figure illustrates, trial activity in China has tripled in less
than a decade, and will increase on the basis of the following developments:
-
Firstly, the [Chinese Food and
Drug Administration (CFDA)] is actively encouraging the conduct of China clinical
studies (including phase I, II and III studies) at the same time as the global
clinical trials program; in the past, China studies were inevitably conducted
after global programs were largely complete; and
-
Secondly, CFDA is actively
accelerating the review of Clinical Trial Applications (CTA) and in the last 24
months the number of approvals has increased from 687 (in 2014) to 3666 (in
2016). This is a five-fold increase in just two (2) years across all
therapeutic areas; we estimate about half of these approvals are in oncology.[84]
3.78
Medicines Australia informed the committee that '[t]he implication of
these developments' is such that:
...China will start to run more clinical trials as part of
global trial programs and that it will recruit quickly. For innovator medicines
companies, which must make decisions about where to place trials in the global
setting, this means that trials will most likely begin to move from slower
and/or more costly markets, to China.[85]
3.79
Ms Elizabeth de Somer of Medicines Australia explained why Australia is
no longer as competitive as other countries as a place to run clinical trials:
...other countries that have entered into the clinical trial
competition, such as China, started off at a lower base than Australia and have
rapidly met and now exceed Australia's standards. Australian standards have
more or less stagnated; we have relied on our quality and we have not improved
our costs and time for setting up and initiating clinical trials. These other
countries have; they have addressed the issues and then exceeded Australia's
benchmark.[86]
3.80
Medicines Australia submitted that the way to overcome these issues
would be to establish 'an Australian Office of Clinical Trials to enable a
national central point of contact to help drive harmonization and quality
standards across the clinical trials sector'.[87]
3.81
Regulatory improvements to clinical trials are discussed later in this
chapter.
Committee view
3.82
The committee is concerned by the barriers to accessing clinical trials
faced by people with LSR cancers, which appear to be more significant for young
people and people in regional and remote Australia. The particular challenges
for young people with LSR cancers are explored in the following chapter.
3.83
The committee is concerned that there is inconsistency in the
availability of trial information for patients through their GPs, and that
patients often resort to "Dr Google" to locate information about
clinical trials. The committee does not discourage patients from researching
possible treatments for their disease, but considers that more could be done to
promote the availability of clinical trial information amongst GPs and the
public more broadly. The committee notes that, in its evidence to the
committee, Cancer Australia conceded that such improvements could be made.
Further discussion about how to increase the awareness of GPs and the public of
LSR cancers appears in chapter 5.
3.84
The Australian government website, AustralianClinicalTrials.gov.au, has
the potential to be a valuable resource to LSR patients and their families.
However, the committee has heard that the website is complex and difficult to
navigate, requiring those searching to be familiar with precise diagnoses and
medical terms. The committee believes that improvements should be made to the
Australian clinical trials site so that is a resource and not a further barrier
to accessing trials. The CCV's clinical trial website, VCTL, which allows the
user to search by cancer type, trial type, phase, molecular target and
hospital, and filter results by gender, age, diagnosis, surgical and medical
treatment(s) already received, is a much more user-friendly and accessible
format. It also provides pop up explanations of medical terms and phrases. In
improving the Australian clinical trials website, the Australian government
should look to the VCTL as an example.
Recommendation 3
3.85
The committee recommends that the Australian government improves
AustralianClinicalTrials.gov.au so it is more accessible and user-friendly.
3.86
The committee appreciates that traditional clinical trial design
deliberately excludes certain patients so that results are rigorous and
replicable. However, patients with LSR cancers are not your "usual"
patients and maintaining the status quo is unacceptable, it is simply hindering
progress towards potential treatments and improvements in survival rates.
Innovative trial designs must be devised and allowed, with appropriate
regulation, to be pursued. The committee welcomes the approach taken by
Professor Thomas of the Garvan Institute; the committee encourages more
researchers to follow this approach where an exclusion criterion is the
availability of other trials.
3.87
While it is not appropriate for the committee or the Australian
government to dictate to researchers their scientific methods and protocols,
the committee expects that the Australian government will address regulatory
barriers which limit the availability of clinical trials for LSR cancer patients.
Regulatory barriers are addressed in detail in the following sections of this
chapter.
3.88
The committee is also deeply concerned by the difference in access to
clinical trials for people with LSR cancer living in regional and remote
Australia, in comparison with people living in metropolitan areas. This is
particularly egregious given LSR cancer patients in regional and remote areas
suffer worse five year survival rates than their metropolitan counterparts.
3.89
The committee welcomes the Australian Teletrial Model and the national
implementation guide issued by COSA.[88]
Teletrials will continue to play an important and hopefully greater role in
facilitating access to clinical trials by LSR cancer patients in regional and
remote areas. However, the committee is of the view that LSR cancer patients in
regional and remote Australia must be assisted to participate in person in
clinical trials.
3.90
The inability of LSR cancer patients participating in clinical trials to
access state and territory patient travel subsidy schemes, and the
inconsistency in the subsidies provided, are further barriers to greater
participation in clinical trials. The committee urges state and territory
governments to consider allowing patients participating in clinical trials to
access patient travel subsidy schemes and to agree on consistent subsidy rates
based on the distance and method of travel, and the average cost of accommodation
in the city in which patients are participating in the trial.
Recommendation 4
3.91
The committee recommends that state and territory governments
consider:
-
allowing low survival rate cancer patients participating in
clinical trials to access patient travel subsidy schemes; and
-
agreeing on consistent subsidy rates based on the distance and
method of travel, and the average cost of accommodation in the city in which
the patient is participating in the trial.
3.92
Finally, in respect of international trials, the committee welcomes the
participation of Australian people with LSR cancers in international clinical
trials, and is encouraged by evidence received about the number of participants
in such trials. The committee acknowledges that not only does this have a
significant impact for the individual involved in the trials, but it may also
lead to ground breaking advances for people with LSR cancers. However, participation
in international trials often comes at great cost to the patient and the
committee considers that more could be done to reduce the financial barriers to
accessing international trials for all LSR cancers. The committee would also
like to see the inclusion of Australian trial sites in collaborative international
trials increase.
Recommendation 5
3.93
The committee recommends that Australian governments improve
access to international clinical trials for people with low survival rate
cancers, including by:
-
exploring ways to reduce the financial barriers to accessing international
trials to the extent possible; and
-
further developing the existing capacity for international
collaboration on trials.
Clinical trials and regulatory issues
3.94
A number of submitters and witnesses raised regulatory issues that
impede access to trials for patients with LSR cancers.
3.95
For example, Mr Peter Orchard of CanTeen Australia observed that the
research being undertaken by individual states and individual hospitals 'is not
always well coordinated and not well shared', and therefore advocated for 'a
national direction to be laid out and national strategies to be laid down and
have funding attached to them, to try and drive changes in behaviour to a more
nationally coordinated approach'.[89]
3.96
The Children’s Cancer Research Unit (CCRU) described a clinical trial it
undertook that took 12 years to be approved.[90]
Professor Jennifer Anne Byrne informed the committee that '[a] lot of the
delays were regulatory delays', explaining that:
We would submit an application. It would go to a body based
in Canberra that would consider it. It would take a long time for us to get
comments back. We would get those comments. We would need to address them. Then
there would be another long period. The regulatory process often involves long
periods of waiting, during which time you could work on certain things in the
laboratory. You can certainly get things ready but you cannot treat a patient.
That is an issue that affects clinical trials but also other kinds of research.[91]
3.97
The following sections examine the most prevalent regulatory issues
raised during the course of the inquiry, namely:
-
barriers to gaining ethics and governance approval;
-
the differences between state and territory jurisdictions;
-
the differences between private and public hospitals; and
-
issues with respect to insurance.
Ethics and governance approval
3.98
Although it acknowledged that 'some changes have been made to streamline
ethics approval processes in Australia' for clinical trial processes, the
Children's Hospital Foundation and Australian Centre for Health Services
Innovation noted that 'governance approval processes remain largely unchanged'.[92]
3.99
The Children's Hospital Foundation and Australian Centre for Health
Services Innovation outlined the process for obtaining ethical and governance
approval for clinical trial research in Australia:
Prior to conducting a clinical trial in Australia, it is
necessary to obtain approval from a Human Research Ethics Committee (HREC) to
ensure that the proposed research will be undertaken in compliance with the
National Statement on Ethical Conduct in Human Research (2007). After obtaining
HREC approval, it is a requirement in most Australian public hospitals and
research institutes to obtain governance approval. Governance approval is based
primarily on resourcing, budget, legal, contractual, insurance and indemnity
issues, and provides approval to conduct the clinical trial under the auspices
of the institution.[93]
3.100
It was further noted that:
Delays in obtaining governance approval of over a year or
more have been reported and primarily result from lack of clarity, consistency
and transparency of governance processes. These avoidable delays in ethical and
governance approvals are themselves unethical. In addition, most institutions
choose to wait until ethics approval is granted before commencing governance
review. It is essential that the role of the research governance office in an
institution be clearly defined and adequately resourced to ensure that approvals
can be issued in a timely manner and patients have access to much needed
treatment. Furthermore, it is important that research institutions take
responsibility for appropriate training and coordination of ethics and
governance submission/re-submission processes including provision of resources
that appropriately support the investigators wishing to undertake research.[94]
3.101
CanTeen advocated for faster approval processes for clinical trials in
hospitals through the introduction of legislation requiring hospitals to be
bound by one ethics process, and changes in the hospital governance process,
noting that:
The fact that you have to go and repeat ethics approvals in
multiple settings and get governance approval in multiple settings can really
slow down the rollout of a trial, and then, if we are talking about
international competitiveness, it does not make us internationally competitive
with the other research markets around the world.[95]
3.102
Speaking of her personal experience with the clinical trial process, Mrs Carly Gray,
whose young son passed away as a result of a diffuse intrinsic pontine glioma
(DIPG), called for a national network of trials across jurisdictions and
collaboration between hospitals and research institutions, asserting that
'[p]atients cannot afford to wait for trials to begin'.[96]
State and territory jurisdictions
3.103
In respect to ethics approval, the NHMRC observed that:
The operation of ethics committees and the approval, conduct
and monitoring of research are the responsibility of the states and territories
that apply both national and state specific guidelines and legislation.[97]
3.104
The NHMRC therefore noted that although it 'is responsible for setting
the national standards for human research in Australia', such as the National
Statement on Ethical Conduct in Human Research (2007)[98]
and the Australian Code for the Responsible Conduct of Research:[99]
The authorisation of human research at a particular
institution (e.g. hospital or university) and the conduct of that research by a
researcher or health practitioner are subject to a variety of national, state
and territory laws and policies.[100]
3.105
This variance in laws and policies across jurisdictions was discussed by
a variety of submitters and witnesses, who noted a lack of consistency between
states and territories with respect to clinical trials.
3.106
In its submission, Medicines Australia recognised that '[t]he systems
under which clinical trial sites in Australia are approved differ between
states and territory' and the possible difference between sites within states
for research governance, is 'an avoidable inefficiency'.[101]
It recommended implementing previous recommendations made to the government,[102]
as well as:
Establishing an Australian Office of Clinical Trials, being a
national coordination unit, to enable a national central point of contact to
help drive harmonization and quality standards across the clinical trials
sector; this would entail working collaboratively with the Commonwealth, States
and Territories[103]
3.107
Medicines Australia also outlined the effects of this on GPs and
patients:
Physicians need to have that real-time ability to find out
where trials are happening for their patients sitting there right in front of
them today. But, because it is fragmented across institutions and
jurisdictions, it is very difficult for them to do that, and, because of the
way that our primary care and our tertiary care operate, they do not have the
time to dedicate to searching for those things.[104]
3.108
The NHMRC outlined the work it has undertaken to streamline clinical trials:
between 2013 and June 2017, $6.3 million was provided to the NHMRC under two
budget measures, Expediting Clinical Trial Reform in Australia and Simplified
and Consistent Health and Medical Research, 'to develop a nationally
consistent approach to clinical trials, improve efficiency and streamline
administration and costs with the aim of positioning Australia as a world
leader in clinical research'.[105]
3.109
A key outcome resulting from this funding was a National Good Practice
Process, piloted at 16 clinical trial sites across all Australian jurisdictions
except the Northern Territory, and intended to streamline clinical trial site
assessment and authorisation phases.[106]
3.110
As part of its work streamlining clinical trials, the NHMRC also noted that
it launched AustralianClinicalTrials.gov.au in 2012, in conjunction with the
Department of Innovation, Industry and Science.[107]
3.111
In examining some of these measures, the CCA and COSA commented that
'[c]urrent governance and ethics requirements are administratively burdensome
and resource intensive, and take considerable time to satisfy'.[108]
It was submitted that the structural barriers to conducting clinical trials—which
the CCA and COSA consider the 'greatest obstacles to conducting clinical trials
in low incidence and low survival cancers', rather than lack of funding—could
be overcome by '[i]mplementing systematic changes to improve collaboration will
support the sustainability of the cancer research sector and translation of
outcomes into practice'.[109]
3.112
Roche Products Pty Limited (Roche) also identified areas where
improvements could be made with respect to streamlining clinical trials. Roche commented
that, in Australia, '[m]any approval systems remain inefficient and manual,
with wide variation and incompatibility between states and even hospitals
within the same state'.[110]
Roche continued:
Governance approval by institutions is often delayed due to
inconsistent requirements, based on a poor understanding of essential and
non-essential steps. These issues are compounded for rarer cancers where the
need to find patients and the lack of treatment centres with expertise may mean
ethics and governance delays have a greater impact.
The need for reform has been recognised by many reviews and
government committees, including the 2013 McKeon Review of medical research.
The Government has committed to addressing competitiveness through an election
announcement of $7 million to improve access to clinical trials in Australia
and through the [Council of Australian Governments] Health Council. Roche
supports urgent action to position Australia as an international research
partner of choice.[111]
3.113
Roche therefore recommended that the Australian government '[i]mplement
regulatory reforms in partnership with state and territory governments to
streamline the clinical trials approval processes'.[112]
3.114
Similarly, the Walter and Eliza Hall Institute discussed the requirement
to obtain multiple ethical approvals across states, and made some
recommendations for harmonising ethics committees and streamlining governance:
The time spent obtaining multiple ethical approvals in order
to put Australian patients with the same disease on the same trial in different
states causes critical delays, with impact on patients’ opportunities to
receive treatment. Harmonisation of human research ethics committees at a
national level should be facilitated. Similarly, governance needs to be
streamlined.[113]
3.115
The NHMRC also noted other activities that it has undertaken in order to
streamline ethics approval:
-
single ethics review/ 'mutual acceptance': the National
Certification Scheme of Institutional Processes related to the Ethical Review
of Multi-centre research commenced in 2010, and NHMRC has certified 44
institutions under this scheme. Additionally, Departments of Health in all
states and territories, bar the Northern Territory and Tasmania, are party to
an Memorandum of Understanding for the National Mutual Acceptance 'of ethics
and scientific review of clinical trials conducted in each of the participating
jurisdictions’ public health organisations', which is restricted to mutual
acceptance between approved state health organisation Human Research Ethics
Committees;[114]
and
-
the Human Research Ethics Application: this replaces the
National Ethics Application Form (NEAF), and aims 'to facilitate efficient and
effective ethics review for research involving humans (i.e. not limited to
clinical trials).[115]
It was adopted by the IT platform currently used by the health systems in New
South Wales, Victoria, South Australia and the Australian Capital Territory
'for the management of ethics review and site approval and authorisation';
however, 'timelines for ethics approval may still vary both within and between
the public and private health sectors'.[116]
3.116
Regardless of these changes, the committee heard that in practice,
ethics approval is not straightforward. For example, speaking to the time it
takes to set up a clinical trial, Mrs Helen Aunedi of Roche informed the
committee that 'it comes down to delays in our budget and contractual
negotiations', noting that there are '200 accredited ethics committees in
Australia'.[117]
Mrs Aunedi advocated for 'a centralised committee that can review and approve
these clinical studies so we can start quicker', but noted that there is also a
delay at the site level, because of the contract, the indemnity and the
insurance:
These are all core templates, so we do not really understand
why the institutions are spending so much time negotiating on these issues. But
I think, simply, if we could fix that aspect, and then we could perhaps use the
national office to promote more of this mutual acceptance. We already have it
in place. We just need to have it at the federal level. So it would be great to
get support from this inquiry to be able to move that forward.[118]
Public versus private hospitals
3.117
The differences between states and territories in respect of ethics
approval and conducting clinical trials also arise in respect of public versus
private hospitals.
3.118
For example, Ms Emma Raymond of Wesley Medical Research informed the
committee that the process for ethics approval in a private hospital is far
simpler than the process in public hospitals:
In the private sector, you know who your ethics committee is.
It is very simple: you know where the forms are, you submit them, and it is
done. If they have any questions they will come and ask you. When it goes
across to the public system, they have a thing called a NEAF, which is supposed
to allow for an easy application—one large application, and then site-specific
applications for each hospital. But it does not work that way. I did a NEAF
that was approved—one site was approved. I used the same documents for another
Queensland Health hospital and we had to rewrite everything ...
A NEAF...has about 61 pages where you answer a lot of questions
and upload documents about the research, and then, for each hospital site that
you want access to, you then have to do another application, which is then looked
at by each hospital's ethics committee. Once it is approved there, it then goes
to the governance committee. The problem arises if you have not filled
something out correctly. At one point I had the wrong number on a page. They do
not tell you that; they just put it on hold and then when they finally get back
to you have to resubmit it again, but you have missed the next deadline for the
ethics committee, so then it gets held over again and then, if it gets to
governance, and they do not like the paperwork, it gets held up again. That is
before you even start the research.[119]
3.119
Ms Raymond also observed that there are different time pressures on
clinicians in public, compared to private, hospitals:
...in the private sector there is more of a focus on clinician
research. In the public sector they are too busy and there are too many people
involved from start to finish. Sometimes, the clinician who is doing the care
will not even know that they have gone on to have treatment because it is just
such a busy, fast-paced scenario.[120]
3.120
Ms Delaine Smith of the ALLG informed the committee that private
institutions 'are traditionally not substantial contributors to investigator
initiated clinical trials', explaining:
There is little to almost no incentive for private facilities
or clinicians to have their patients participate in clinical trials. This
impacts adversely on the rate of patient accrual to clinical trials. The second
point is that, additionally, there is no incentive or support from private
health insurers to have their patients participate in clinical trial
research—it is simply not there. One could argue that it is even a greater
priority for the private sector to participate and champion research that
inevitably will have the potential to bring about healthcare efficiencies and
cost savings.[121]
3.121
Professor Andrew Roberts, also of the ALLG, provided a further
explanation:
It is quite clear that to be
involved in a clinical trial requires extra care, extra time, extra resources
and therefore extra costs. Clearly that affects issues around reimbursement,
whether that is through private or government. Ultimately, to participate in
clinical research, the patient, the doctor, the sponsor of the trial and our
health system are invested, and it is a question of whether they are clear
about that and whether there is an alignment of purpose.[122]
3.122
The ALLG suggested that the way in which to overcome the obstacle that
clinicians are time poor, which can impact matters such as timely access to
information about clinical trials, could be to encourage models that encourage
public/ private partnerships.[123]
The ALLG also recommended enabling collaboration between public and private
institutions by engaging with insurance companies and the private health care
sector, and implementing 'national clinical trial uptake across public and
private hospitals' as ways to improve survival rates by establishing Key
Performance Indicators (KPIs) for hospitals regarding clinical trial
participation, their uptake of patients to clinical trials, and creating 'a
culture of positive benefit'.[124]
3.123
CanTeen Australia also proposed collaboration across institutions,
recommending the establishment and operation of national low survival cancer
trial networks which would:
...operate across multiple hospital boundaries (including
across local health districts, public and private hospitals and adult and
paediatric settings), assure rapid trial initiation, consistent, cost effective
and timely ethics, governance and other relevant approvals, rapid and targeted
access to patients and consistent monitoring processes and standards.[125]
Insurance
3.124
In evidence to the committee, CanTeen highlighted the importance and
benefits of exploring options around a national insurance scheme covering
clinical trials which would alleviate the burden that individual hospitals
currently face by having to seek coverage for a given trial:
... in terms of insurance: again, could there be a national
insurance scheme that covers trials so that we do not have this business of
every hospital having to go to see whether their particular insurer will cover
them for this trial?
Just in terms of that insurance process alone: that gets
replicated in every hospital, let alone them needing to ask about the impacts
on their staffing or their budget. It is an understandable process that they
have to do, but to take four or five months for it is the part that does not
seem to be valid, really. If we are really keen about getting patients into
trials quickly and getting good research happening, we need to make those times
shorter.[126]
3.125
In response to this suggestion, Professor Anne Kelso of the NHMRC stated
that it was outside of the NHMRC's remit to do such work, and that 'unless we
were tasked and funded to do a particular project; it's otherwise not within
the remit of NHMRC's activities'.[127]
Committee view
3.126
While there have been recent changes to improve streamlining of clinical
trial ethics approval, the evidence presented to the committee indicates that differences
in ethics and governance approval processes between states and territories, and
private and public hospitals continue to delay and in some instances discourage
trials or trials across multiple sites.
3.127
The committee welcomes suggestions from various submitters and
witnesses, such as removing the requirement to obtain ethics and governance
approval for each individual trial site; the establishment of an Australian
Office of Clinical Trials to be a national coordination unit and national
central point of contact to help drive harmonization and quality standards;
further regulatory reforms to streamline approvals processes; and facilitating
better collaboration between private and public institutions.
3.128
The committee recommends that Australian governments address the
remaining barriers arising from differences in ethics and governance approval
processes as a matter of priority, and in doing so give serious consideration
to the proposals recommended to this inquiry.
Recommendation 6
3.129
The committee recommends that Australian governments, as a
priority, further streamline ethics and governance approval processes for
clinical trials, particularly where those processes differ between states and
territories, and public and private research institutions.
3.130
Further, the committee acknowledges the work that the NHMRC has done to
reduce unnecessary regulatory barriers with respect to ethics processes, and
while it recognises that some processes are beyond the scope of the NHMRC, the
committee considers that the NHMRC could make further changes in order to
eliminate those existing, significant regulatory delays.
3.131
Specifically, the committee considers that the NHMRC could develop a
standard template and associated guidelines, including timeframes, for ethics
and other governance approvals that could be adopted by every state and
territory. This in turn could allow for the approval from one institution to
lead to automatic approval at any other institution.
Recommendation 7
3.132
The committee recommends that the National Health and Medical
Research Council develops a standard template and associated guidelines,
including timeframes, for ethics and other governance approvals for
consideration and possible adoption by each state and territory.
Navigation: Previous Page | Contents | Next Page