Introductory Info
Date introduced: 24 March 2021
House: House of Representatives
Portfolio: Health
Commencement: Sections 1 to 3 commence on Royal Assent. Schedule 1 commences on the earlier of Proclamation or six months after Royal Assent.
The Bills Digest
at a glance
Mitochondrial disease is a group of
conditions that can cause serious health issues and, in severe cases, can cause
death in childhood. There is no known cure for mitochondrial disease.[1]
Mitochondrial donation is an assisted reproductive
technology (ART) that can assist women to avoid passing mitochondrial DNA
disease to their biological child. This technology is not a cure for
mitochondrial disease but is rather a way to prevent children from inheriting
mitochondria that can cause mitochondrial disease.[2]
Under the current legislative framework, mitochondrial
donation is illegal under the Prohibition of Human Cloning for
Reproduction Act 2002 (Cth) and the Research Involving Human
Embryos Act 2002 (Cth). The Mitochondrial Donation Law Reform (Maeve’s
Law) Bill 2021 (the Bill) amends relevant Acts and associated Regulations to make
mitochondrial donation legal for research, training and human reproductive
purposes. The overall aim is for women at risk of passing on mitochondrial
disease to have reproductive options for biological children without the
increased risk of their child having mitochondrial disease.
Primarily the Bill makes changes to ensure that it is no
longer an offence to create, for the purposes of reproduction, and under
the relevant mitochondrial donation licences, a human embryo that:
- contains
the genetic material of more than two people and
- contains
heritable changes to the genome.
Given mitochondrial donation is a new medical technology, the
Bill introduces it in a staged and controlled manner with a two-stage implementation
approach. This is intended to allow for the expansion of scientific evidence to
ensure the techniques are safe and effective and undertaken in an ethically
appropriate manner.[3]
The Bill introduces five types of mitochondrial donation licences:
- a pre-clinical research and training licence
- a clinical trial research and training licence
-
a clinical trial licence
- a clinical practice research and training licence and
-
a clinical practice licence.
Initially, only three of the potential five licences will
be available from the National Health and Medical Research Council (NHMRC). These
initial licences would authorise pre-clinical and clinical trial research and
training and clinical trial activities to take place. This would enable the
scientific evidence to continue to expand and would also allow ‘the safety,
efficacy and feasibility of mitochondrial donation for reducing the risk of
transmission of serious mitochondrial disease in humans’.[4]
The Bill contains provisions detailing what is authorised
under each of the mitochondrial donation licences, with provisions relating to
licence applications, conditions and administrative requirements. The licences
will be administered and regulated by the Embryo Research Licensing Committee of
the NHMRC (referred to in this Digest as the NHMRC Licensing Committee), with
dedicated provisions for the close vetting and oversight of applicants and
licence holders by the NHMRC.
Stage 2, which would permit mitochondrial donation in
clinical practice, is dependent on the success of the licenced techniques and
the findings of the Stage 1 review. As such, the two clinical practice related
licences are subject to further amendments to legislation before they can be
made available.
Whilst at the time of writing there has been limited
commentary since the introduction of the Bill, there have been robust
discussions through different consultation processes. Respondents have
primarily focussed on the ethical and social considerations and it has also
been widely noted that this is a new scientific technology. Due to the complex
and sensitive nature of the subject, people who have engaged with the
consultation processes appear to hold views that tend to be heavily skewed either
for or against.
In acknowledgement of the ethical issues and concerns raised
by the new technology endorsed by this Bill (including privacy, embryo creation
and destruction, consent and donor rights) the Minister for Health has stated
that the Bill will be put to a conscience vote.[5]
Purpose of the Bill
The purpose of the Mitochondrial
Donation Law Reform (Maeve's Law) Bill 2021 (the Bill) is to introduce mitochondrial donation techniques in a staged and
cautious approach, initially through research and a clinical trial. Following a
review of Stage 1, and further legislative changes, mitochondrial donation could
be made available in the clinical practice setting. Once more widely available
it is estimated that this technology could prevent 60 babies being born with
mitochondrial disease each year in Australia.[6]
The Bill:
- provides
for mitochondrial donation techniques to be administered under five types of
mitochondrial donation licences. Only three of the five licences would be
available in Stage 1, which would allow pre-clinical and clinical trial
research and training and clinical trial activities to take place
- provides
for the licences to be administered and regulated by the NHMRC Licensing
Committee
- sets
out provisions dealing with what is authorised under each of the five types of
mitochondrial donation licences, with strict conditions relating to licence
applications, conditions and administrative requirements. In addition, licence
holders would be subject to additional, and ongoing, requirements
- Stage
1 will allow eligible women to access mitochondrial donation by participating
in the clinical trial. It is anticipated that a low number of people will
access the technology, and the women who do participate, may require multiple
rounds of IVF. As such, the trial is expected to take place over approximately
10 to 12 years.[7]
The following legislation is engaged and amended to give
effect to the purpose of the Bill:
Structure of
the Bill
The Bill comprises a single schedule of three parts:
- Part
1 contains the main amendments to the PHCR Act, RIHE Act and
the RIHE Regulations.
- Items 4 and 5 of
the Bill makes the most significant amendments to the PHCR Act by
changing the legislation to ensure that it is no longer an offence to allow,
under a mitochondrial donation licence, the creation of an embryo with:
- the
genetic material of more than two people (item 4)
- changes
to its genome that would be heritable by the child’s descendants (item 5).
- Item 17 of the Bill makes the bulk of the amendments to the RIHE Actby adding proposed Division 4A
of Part 2, comprising of:
- Subdivision
A—Kinds of mitochondrial donation licences and what they authorise
- Subdivision
B—Applying for a mitochondrial donation licence
- Subdivision
C—Determining applications for mitochondrial donation licences
- Subdivision
D—Conditions of mitochondrial donation licences
- Subdivision
E—Ongoing requirements for holders of mitochondrial donation licences
- Subdivision
F—Variation, suspension, revocation and surrender
- Part
2 of the Bill contains other amendments to the PHCR Act, RIHE Act,
FOI Act, RIHE Regulations and the Therapeutic Goods (Excluded Goods)
Determination. These amendments will support the main amendments in Part 1.
- Part
3 of the Bill contains application and transitional provisions for the RIHE
Act and the RIHE Regulations.
A detailed explanation of Part 1 is
outlined on pages 4 and 5 of theExplanatory
Memorandum to the Bill.[8]
Background
Scientific background
Mitochondria
and mitochondrial DNA
Mitochondria are small DNA-containing structures found in
human cells. Mitochondria produce roughly 90 per cent of the energy our body
needs to function and also support a number of other functions.[9]
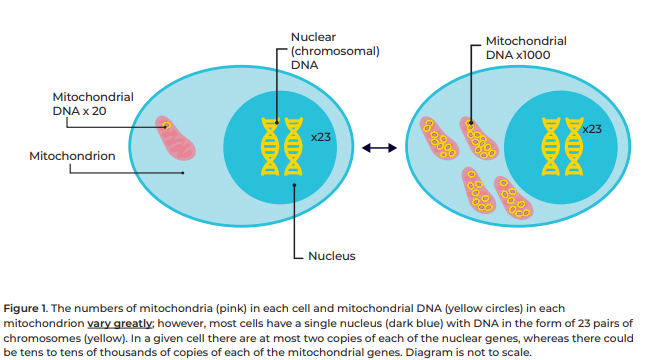
Mitochondrial DNA (mtDNA)
differs from nuclear DNA (nDNA) in a variety of ways, a few of which include:
- mtDNA
contains 37 genes, nDNA contains 20,000 to 30,000 genes
- mtDNA
is inherited primarily from the biological mother due to the mitochondria being
present in the mother’s egg cell,[10]
nDNA is inherited from both biological parents
- a
single cell can contain numerous mitochondria, and each of these can contain numerous
copies of mtDNA, a single copy of nDNA is contained in the nucleus of most cells
and there is usually just one nucleus per cell[11]
- mtDNA
has a much higher mutation rate than nDNA, estimated to be 100 times higher.[12]
Figure 1 below provides an illustration of nDNA and mtDNA
within a cell.
Figure 1: illustration of nDNA and mtDNA within a cell
Source: NHMRC, Mitochondrial donation issues paper: ethical and social
issues for community consultation,
NHMRC, [Canberra], [2019], p. 7.
Mitochondrial
DNA disease
Mitochondrial DNA disease refers to a mixed (heterogenous)
group of inherited conditions caused by mutations in mtDNA or nDNA that impact
the function of mitochondria (that is, reduce their ability to produce energy) and
can cause serious illness. This results in cell injury and death and can lead
to whole organ systems failing, which can be fatal.[13]
Currently, there is no known cure and treatment is mostly limited to the management
of symptoms.[14]
It is estimated that approximately:
- one
in 200 Australian babies are born with some level of mtDNA mutation that could lead
to mitochondrial DNA disease in their lifetime, although for many, the levels
of mutated mtDNA are too low to cause disease. This is estimated to be roughly
120,000 Australians[15]
- between
one in 5,000 and one in 10,000 Australians are estimated to develop severe or
life‑threatening mitochondrial DNA disease during their lifetime
- the
average lifespan of children born with mitochondrial DNA disease is estimated
to be between three and 12 years of age. However, the (symptomatic) onset of the
disease can affect people at any age and some individuals do not develop
symptoms until adulthood.[16]
[emphasis added]
Mitochondrial DNA disease can cause a lot of different
symptoms because mitochondria are located everywhere within the body. Due to
their role in energy production, the disease particularly affects organs that
have a higher energy use, such as the heart, muscles and brain. The proportion
of unhealthy mtDNA (referred to as mutation load) may affect the severity of the
mitochondrial DNA disease, for example, a low level of mutated mtDNA may mean
that a person does not exhibit any symptoms.[17]
Because one cell can contain many mitochondria, this can result in
heterogeneous mtDNA within the same cell. When the cell divides the
mitochondria are split between the two daughter cells in a random manner
(unlike nDNA).[18]
As a result, a cell can have a mix of healthy and unhealthy (or mutated) mtDNA
(see Figure 2).[19]
Figure 2: the mixed nature of healthy and mutated mtDNA within cells

Source: NHMRC, Mitochondrial donation issues paper, op. cit., p. 9.
For women who are heterogeneous carriers of mitochondrial
DNA disease (that is, they have both healthy and mutated mtDNA) it can be
difficult to predict the impact on a future child. This can leave people facing
difficult decisions on whether to have children and, if they do conceive, whether
to seek prenatal testing (if that is an option).[20]
Prenatal testing is discussed further under the ‘Treatment and prevention’ and
‘options for having children’ sections on pages 22–24.
Mitochondria
donation
Mitochondrial donation is an assisted reproductive
technology (ART), involving in-vitro fertilisation (IVF).[21]
IVF refers to procedures where human eggs, sperm or embryos are handled outside
of the body (in-vitro) in order to establish a pregnancy.[22]
The purpose of mitochondrial donation is to reduce the risk of a child
inheriting mitochondrial DNA disease from a woman carrying the condition.[23]
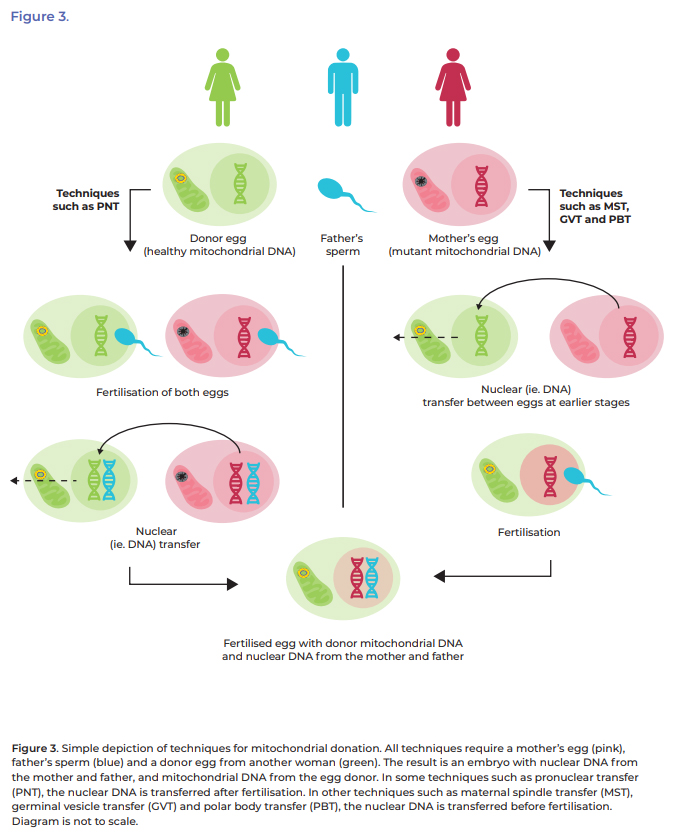
This is achieved by creating an embryo using nDNA from the
prospective mother and father and healthy mtDNA from a donor. Several different
mitochondrial donation techniques can achieve this outcome, including:
- maternal
spindle transfer (MST)
- pronuclear
transfer (PNT)
- polar
body transfer (PBT) and
- germinal
vesicle transfer (GVT).[24]
Pages 42 and 43 of the Explanatory Memorandum to the Bill provides
a detailed definition for each of these techniques.[25]
A simplified overview is given below in Figure 3.
Figure 3: simplified overview of mitochondrial donation techniques
Source: NHMRC, Mitochondrial donation issues paper, op. cit., p. 13.
Mitochondrial donation is a relatively new technology and
the long-term consequences are still unknown.[26]
Other options for having children are available to women that carry mtDNA
mutations, these are discussed further under the ‘Options for having children’
section on pages 22 to 24 of the Bills Digest.
It is important to note that mitochondrial donation cannot
cure existing mitochondrial DNA disease or prevent mitochondrial DNA disease
caused by mutations in nDNA. Estimates suggest mitochondrial donation may be
able to assist in the prevention of mitochondrial DNA disease in 60 births per
year in Australia.[27]
If mitochondrial donation becomes part of clinical practice in Australia, it is
expected that only a small number of women will access the technique.[28]
Policy background
Current legislation
RIHE Act
and PHCR Act
The Research Involving Human Embryos Act 2002 (RIHE Act) and the Prohibition
of Human Cloning Act 2002 were passed by Parliament in December 2002 in
response to community concerns, including ethical ones, about scientific
developments in relation to human reproduction and the use of human embryos. Both
Acts were amended in 2006 and the Prohibition of Human Cloning Act 2002 was
renamed the Prohibition of Human Cloning for Reproduction Act 2002 (PHCR
Act).[29]
The Acts establish a regulatory framework that prohibits
certain practices, including human cloning, and regulates the uses of excess
human embryos created through ART.[30]
Intergovernmental
agreement and state and territory legislation
In March 2004, the Council of Australian Governments
(COAG) agreed to the Research
Involving Human Embryos and Prohibition of Human Cloning for Reproductive
Purposes Intergovernmental Agreement (the IGA). The IGA creates a
nationally consistent scheme for the regulation of research involving human
embryos, the prohibition of human cloning and other unacceptable practices.[31]
Under the IGA all of the states and territories (other
than the Northern Territory) have introduced legislation that is consistent
with the Commonwealth’s RIHE Act and PHCR Act.[32]
Due to the staged approach and the way the Bill proposes introducing
mitochondrial donation, namely for very limited purposes restricted by the
proposed mitochondrial donation licences, the amendments introduced by the Bill
will not need to form any part of the nationally consistent scheme under the
IGA. This allows mitochondrial donation to be legalised under those limited circumstances
without corresponding amendments needing to be made to state or territory law
for Stage 1.[33]
International
policy
In 2015, the United Kingdom (UK) passed legislation to
legalise mitochondrial donation using the PNT and MST techniques. Prior to this,
the UK amended legislation to allow researchers to develop mitochondrial
donation techniques and conducted multiple reviews of the science as well as public
consultations on the social and ethical issues. To date, the UK is the only
country that has changed its laws and regulations to allow mitochondrial
donation.[34]
Despite being legal, access to mitochondrial donation in
the UK is tightly regulated, approved on a case-by-case basis and only approved
for patients at high risk of transmitting mutations that will lead to serious mitochondrial
DNA disease. At the time of writing, only one facility has been licenced to provide
mitochondrial donation, and the licence only allows for the use of PNT. As of
November 2020, it has been reported that up to 21 couples have attained a
licence to receive treatment and up to eight treatments have been approved. The
outcomes of these treatments have not been made publicly available for privacy
reasons.[35]
Other countries such as the United States, Singapore and
Sweden have released reports or conducted consultations regarding mitochondrial
donation, but legislation has remained unchanged. In countries that have no
specific government legal restrictions on mitochondrial donation techniques,
such as Mexico and Ukraine, there are reports of mitochondrial donation having
taken place.[36]
Australia’s
inquiry and consultations
Senate
Community Affairs Committee inquiry
In 2018, the Senate Community Affairs References Committee
(Senate Committee) undertook an inquiry into the Science of Mitochondrial Donation and Related Matters.
Among other things, the inquiry examined the impact of mitochondrial disease on
Australian families and the healthcare sector, the safety and efficacy of
existing donation techniques, and the ethical considerations.[37]
The Senate inquiry received 60 submissions and held one
public hearing. The Committee’s final report explores the experience of people
with mitochondrial DNA disease and their families, the science of mitochondrial
donation and the ethics and regulation of mitochondrial donation.[38]
The final report made four recommendations to Government:
- undertake
public consultation on the possible introduction of mitochondrial donation into
Australian clinical practice
- obtain
expert advice through the National Health and Medical Research Council (NHMRC)
about key scientific questions relating to mitochondrial donation
- engage
with state and territory governments on the findings of the inquiry and
- as
an interim measure, initiate dialogue with the relevant authorities in the UK
to facilitate access to the UK treatment facility for Australian patients seeking
mitochondrial donation.[39]
Governments
response to the Senate Committee’s report
The Government tabled a response to the Senate Committee’s
report in February 2019. The Government supported the Senate Committee’s
recommendations for expert advice and further consultation activities with the
Australian public and state and territory governments. Regarding opening a
dialogue with the UK for the potential access of Australians to their
mitochondrial donation services, the Government stated that it will reconsider this
recommendation following the consultation outcomes and expert advice.[40]
The Government tasked the NHMRC to establish a panel of
scientists and other experts to provide advice on the legal, regulatory,
scientific and ethical issues identified by the Senate inquiry. The work was
intended to develop the key questions to inform a community-wide consultation.[41]
NHMRC
Mitochondrial Donation Expert Working Committee
In March 2019, the NHMRC convened a Mitochondrial Donation
Expert Working Committee (NHMRC Expert Committee) to examine the questions posed
by the Senate Committee. In June 2020, the NHMRC Expert Committee statement
was released, reporting on three specific questions from the Senate inquiry.[42]
The Chair of the Expert Committee stated:
The Committee agreed that, given the complexity of the issues
raised, some disagreement was both to be expected and acceptable. As such, the
Expert Statement reflects the consensus view of the Committee on each question
as far as was possible.[43]
NHMRC
community consultations
Between September and November 2019, the NHMRC undertook
community consultation on the social and ethical considerations of the possible
introduction of mitochondrial donation in Australian clinical practice.[44]
The consultation process included:
- the
release of a Mitochondrial
Donation Issues Paper to support and inform the consultation process[45]
- a
citizens’ panel composed of ordinary citizens who engaged with mitochondrial
donation experts and stakeholders to learn more about the issues and develop a
position statement
- an
online submission portal to allow people to submit their written views on the
topic
- two
NHMRC hosted webinars discussing the topic
- public
forums held in Sydney and Melbourne and
- targeted
roundtable discussions for key stakeholders to present their views and discuss
perspectives.[46]
In June 2020, the NHMRC published the Community
Consultation Report that discussed the outcomes of the consultation
process.[47]
From the online submissions, the report identified five major themes, outlined below
in Figure 4.
Figure 4: outline of the themes from the community consultation online
submissions
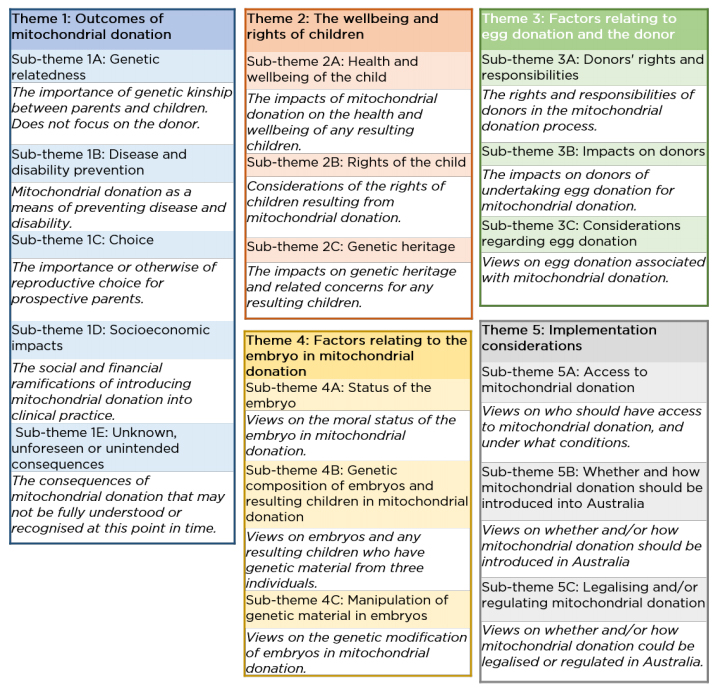
Source: NHMRC, Mitochondrial donation community consultation report, NHMRC, 2020, p. 21.
The Community Consultation Report concluded:
It is clear that there is a range of opinions in the
community about mitochondrial donation, with a number of respondents being
passionately opposed to its introduction while others are supportive. This
range of views must be taken into consideration in any future work on this
issue.[48]
Department
of Health consultation
The Minister for Health announced, on 5 February 2021,
that the Department of Health (DoH) had commenced a consultation process on a proposal to
introduce mitochondrial donation using a two-stage process (discussed further
below).[49]
The public consultation took place from 5 February 2021 to 15 March
2021 and received 74 survey responses and 27 written submissions. The DoH released
its Consultation Summary Report on 22 March
2021. The report found that the majority of the feedback reflected the responses
provided through the Senate inquiry and NHMRC consultation process.[50]
Embryo
research licensing
The PHCR Act and the RIHE Act establish a
regulatory framework around research and the creation, and use of human embryos
through ART. The RIHE Act and the PHCR Act outline arrangements
between the Commonwealth and the states and territories. Both Acts prohibit
particular activities while identifying some activities that can only be
undertaken if authorised by a licence.
The NHMRC Licensing Committee is a Principal Committee of
the NHMRC and is established under Division 3 of Part 2 of the RIHE Act. It
is responsible for overseeing the RIHE Act and the PHCR Act.[51]
To undertake research on human embryos, including use of excess ART embryos and
research and training involving fertilisation, the researcher must operate
under the approved activities of a licence issued by the NHMRC Licensing
Committee.[52]
Tabling of
Reports
The RIHE Act requires the NHMRC Licensing Committee
to table reports to either House of Parliament twice a year, upon request or at
its discretion, about the operation of the Act and the licences issued.[53]
Maintaining
a Licence Database
Section 29 of the RIHE Act requires the NHMRC
Licensing Committee to maintain a public database on each licence, including
information on:
- the
name of the licence holder
- the
number of excess ART embryos or human eggs authorised for use by the licence
- a
short statement of the nature of use of these excess embryos or eggs, and
creation or uses of other embryos, authorised by the licence.[54]
Regulation
of Excess ART Embryos
Part 2 of the RIHE Act regulates the use of excess
ART embryos, other embryos and human eggs. Section 9 provides the following
definition of excess embryos for this Part:
excess ART embryo means
a human embryo that:
- was
created, by assisted reproductive technology, for use in the assisted
reproductive technology treatment of a woman; and
- is excess to the needs
of:
- the woman for whom it was
created; and
- her spouse (if any) at
the time the embryo was created.
(2) For
the purposes of paragraph (b) of the definition of excess
ART embryo, a human embryo is excess to the needs of the persons
mentioned in that paragraph at a particular time if:
- each such person has given written authority for use of the embryo for a purpose other than a purpose relating to the assisted reproductive technology treatment of the woman concerned, and the authority is in force at that time; or
- each such person has determined in writing that the embryo is excess to their needs, and the determination is in force at that time.[55]
Human
Research Ethics Committees
In considering a licence application, the NHMRC Licensing
Committee must be satisfied of a number of criteria, including the proposed
project/activities having been assessed and approved by a Human Research Ethics
Committee (HREC).[56]
Research institutions, such as hospitals and universities, have a HREC that reviews
all research proposals that would involve human participants to ensure the
proposed work is ethically acceptable. The NHMRC has published the National
Statement on Ethical Conduct in Human Research (National Statement), which
sets out the requirements of a HREC and the researchers.[57]
Proposed
two-stage process
The introduction of mitochondrial donation in Australia
has been framed as a two-stage implementation process. The two-stage approach
intends to provide a way for the mitochondrial donation technology and
procedures to be introduced cautiously, with time allowed to gather evidence on
the safety, efficacy and clinical utility of the techniques.[58]
A brief overview of the two-stage approach is provided in Table
1 below.
Table 1: proposed two-stage process for the introduction of mitochondrial
donation
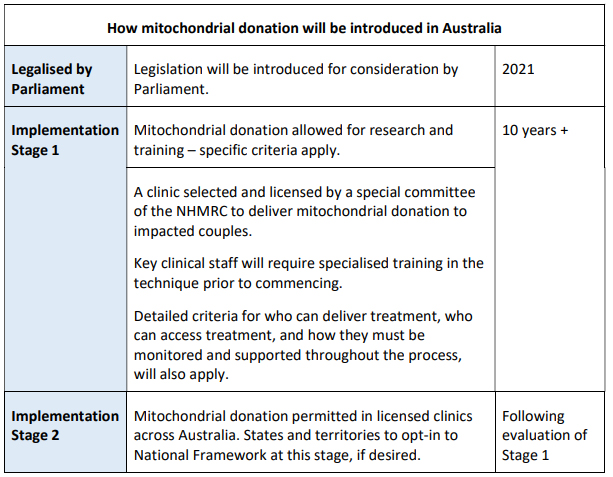
Source: DoH,
Legalising Mitochondrial Donation in Australia: Public
Consultation Paper, DoH, [Canberra],
[2021], p. 5.
Stage
1—research, training and clinical trials
It is proposed that Stage 1 would include:
- an
expansion of the role and remit of the NHMRC Licensing Committee to include
licensing and oversight of specific mitochondrial donation licences
- the
NHMRC Licensing Committee developing the administrative requirements and
assessment procedures for individuals and organisations applying for one of the
following three licence types which permit mitochondrial donation in Australia:
- pre-clinical research and training licence
- clinical
trial research and training licence and
- clinical
trial licence
- the
NHMRC Licensing Committee and the NHMRC developing monitoring processes for
licence holders
- the
DoH running a competitive grants process to identify a suitable organisation to
undertake the clinical trial
- this
trial is anticipated to take approximately 10 years, as the number of
participants is expected to be low and each participant may require multiple
IVF procedures before a successful pregnancy is achieved.[59]
Transition
from Stage 1 to Stage 2
To facilitate the transition to Stage 2, the Bill amends
the PHCR Act and the RIHE Act to establish all five licences,
including the two clinical practice related licences. However, organisations
will not be able to apply to the NHMRC Licensing Committee for either of the
two clinical practice licences until a particular technique is specified in the
RIHE Regulations for this purpose. The permitted
mitochondrial donation techniques for clinical practice will not be authorised
until Stage 1 is completed and the safety and efficacy of the techniques have
been demonstrated.[60]
The states and territories are responsible for the
regulation of ART in their jurisdiction. When undertaken in a clinical practice
setting, mitochondrial donation is expected to be categorised as an assisted
reproductive technology. If Stage 2 goes ahead, mitochondrial donation for the
purposes of clinical practice will not be able to occur in a state or territory
until the relevant state or territory legislation is amended to allow it.[61]
According to the Explanatory Memorandum of the Bill, the
transition to Stage 2, and the specification of the permitted techniques in the
Regulations, will be decided by the Government following consideration of the
progress and outcomes of Stage 1, and other expert advice.[62]
Stage
2—clinical practice
Under Stage 2, it is proposed that:
- jurisdictions
may choose to be part of a national regulatory framework, which will allow for
licenced clinical practice, in participating states or territories
- as
part of the regulatory scheme, the following two licences, to be overseen by
the NHMRC Licensing Committee, would be available:
- the
clinical practice research and training licence and
- the
clinical practice licence.[63]
Ethical and
social considerations
This Bill will be put to a conscience vote.[64]
The Minister for Health outlined the reasoning behind this decision in his
second reading speech:
Some members of the community … have raised concerns about
issues such as privacy of parents and children, creation and destruction of
embryos, ensuring informed consent, donor rights and the newness of the
science. I acknowledge that not all members of the community are comfortable
with the use of this technology, and that's reflected in the free vote—the
ability to vote with conscience—that has been called for jointly by the major
parties.[65]
The NHMRC Expert Committee Issues Paper states:
In addition to questions around safety and effectiveness,
scientists, researchers and others have identified ethical and social issues
that arise from the use of mitochondrial donation in research and in clinical
treatment. These include the rights of the child, the status of the embryo, the
role and rights of women donating eggs, and community considerations.[66]
It is these considerations that appear repeatedly in the submissions
and reports from the Senate inquiry, the NHMRC community consultation
activities and in the summary report on the most recent consultation undertaken
by the DoH. These are complex and complicated issues and ones that people have
strong and opposing views on. This section briefly summarises some of the key ethical
and social considerations raised in the different consultation undertaken.
Treatment
and prevention
There is no cure for mitochondrial disease and no single
treatment option, instead treatment is tailored to the individual.[67]
It is estimated that approximately one in every 200 children born in Australia has
some level of mutation in their mitochondria with between one in 5,000 and one
in 10,000 Australians developing severe mitochondrial disease.[68]
In 2006 a paper was released in The Lancet that suggested that at
least 3,500 women in the UK, most of child-bearing age, were likely to be
carriers of mtDNA mutations which could be passed on to their children,
potentially causing mitochondrial disease.[69]
A review undertaken in 2012 provided some additional context on the estimated
figures for the UK:
However, bearing in mind the limiting factors listed above, a
widely-quoted figure (approximated from different published papers) is that
around one in 6,500 children is thought to develop a more serious mitochondrial
disorder, where some of these disorders can be fatal. In the context of
neuromuscular disease, this figure would make mtDNA disorders one of the most
common inherited neuromuscular disorders.[70]
In a submission to the Senate inquiry in 2018, Professor
Christodoulou, a clinical geneticist, stated:
There are currently very few effective treatments for this
group of disorders, and so prevention is the main approach that can be
currently offered to families. For nuclear-encoded gene mutations traditional
prenatal testing or pre-implantation genetic diagnosis techniques are very
effective and have a long and established track record. However, apart from a
few specific exceptions, for primary mitochondrial DNA disorders these
approaches are generally not as definitive for a number of reasons. It is for
this latter group of disorders where mitochondrial donation is particularly
relevant.[71]
In the NHMRC community consultation, concerns were raised,
including by people with mitochondrial disease, that the introduction of
mitochondrial donation could have negative impacts and could exacerbate
‘negative social perceptions and treatment of people with a disability’.[72]
Options for
having children
There are three main non-medical options available for perspective
parent/s who are at increased risk of passing on mtDNA mutations that could
cause mitochondrial disease (see Figure 5):
- adoption
- fostering
and
- natural
conception.[73]
There are also three main medical options currently
available (see Figure 6):
- prenatal
testing
- pre-implementation
genetic testing and
- use
of a donor egg.[74]
As identified in Figures 5 and 6 below, there are advantages
and disadvantages for all options, including some medical options not being clinically
appropriate for all women. According to the Senate Committee’s final report, some
submissions that did not support mitochondrial donation argued in favour of non-genetic
options.[75]
For some people, having a child who is genetically related
to them is extremely important but this desire is not always considered a
compelling enough reason to introduce mitochondrial donation by people and
organisations who made submissions to the NHMRC and Senate inquiry.[76]
In the (US) National Academies of Sciences, Engineering, and Medicine (NASEM)
report on the ethical, social and policy considerations of mitochondrial
donation, the authors conclude that the current options available achieve some
of the desirable attributes of mitochondrial donation but not all of them.[77]
The Senate Committee expressed the view that it is ‘desirable for governments
to support fertility treatment as a social good’ but this is not an unlimited
responsibility and any treatments should be provided equitably.[78]
The range of options available, which may include
mitochondrial donation, provide people with different choices, with some
options appealing to some people and not others. Importantly, for people to
make these choices, they need readily available access to appropriate
information and support to assist them in making their decisions.
Figure 5: current non-medical reproductive options for women where there is a
risk of transmitting mitochondrial disease to offspring
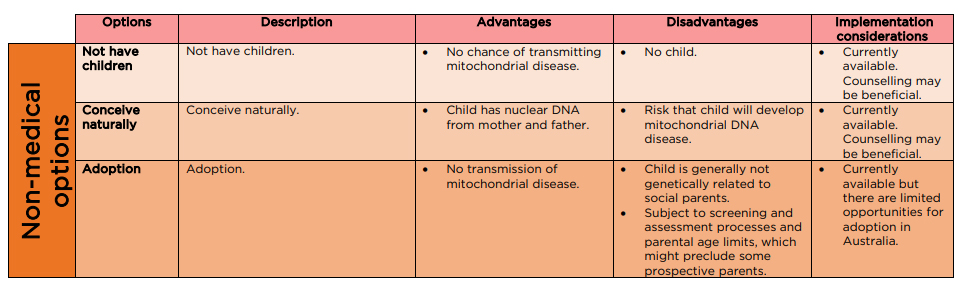
Source: NHMRC Mitochondrial Donation Expert Working Committee, Expert
Statement, NHMRC, [Canberra], 2020, p. 66.
Figure 6: current reproductive options for women where there is a risk of
transmitting mitochondrial disease to offspring
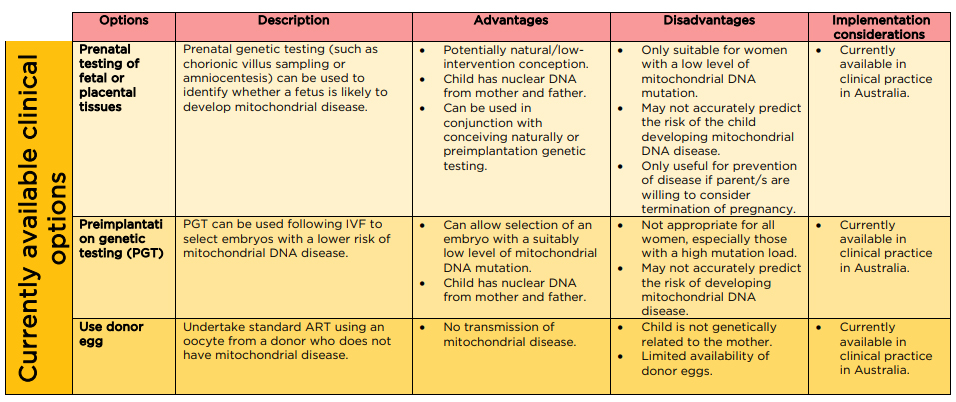
Source: NHMRC Mitochondrial
Donation Expert Working Committee, Expert statement, NHMRC, [Canberra], 2020, p. 67.
Use of new
techniques
As outlined in the scientific background, research on the safety
and efficacy of mitochondrial donation techniques is continuing with new
evidence emerging and with knowledge gaps remaining. Through the community
consultation process undertaken by the NHMRC, the unknown, unforeseen or
unintended consequences were considerations identified in submissions for proponents
and opponents of mitochondrial donation.[79]
Sex
selection
Sex selection through the use of ART is permitted in
Australia but is an option reserved for cases that would reduce the risk of
transmission of a genetic condition or disease that would severely impact the
person’s quality of life.[80]
Sex selection for male embryos with mitochondrial donation is considered for
two different reasons.
As outlined in the Senate inquiry, some experts have
suggested the children from women who were themselves born using a
mitochondrial donation technique may have an additional risk of developing
mitochondrial disease than the children of men born using mitochondrial
donation due to mtDNA carryover.[81]
Sex selection is also raised in consideration of inheritable
genetic modifications. As outlined in the scientific background, mtDNA is
inherited almost exclusively through the maternal line. Therefore, if a man was
born using mitochondrial donation, any child he would have would inherit the
mother’s mtDNA and not his mtDNA. As such, the changes to his mtDNA will not be
passed on to the next generation.
In the NASEM report, it is recommended that mitochondrial
donation be limited to male embryos in the first instance to avoid heritable
genetic modifications. The authors argue this position is not about the
acceptability of sex selection and is rather based on the need to proceed slowly
and prevent potential adverse heritable changes being passed to future
generations.[82]
The Nuffield Council on Bioethics, based in the UK, raise concerns about the use
of sex selection with mitochondrial donation and provide the following extract
from a submission it received during its consultation:
In suggesting that only males be conceived initially, there
is an underlying assumption that the unknown long-term adverse consequences
would relate only to the mitochondria (passed to the next generation through
eggs, not sperm). As this may not be the case, there is no justification for
limiting the risk to one particular sex. No technique for the eradication of
disease should be permitted until there is reasonable evidence for its safety.
We would not argue that experimental treatments should not be permitted in
medicine, however… if such a course of action as selecting only males needs to
be considered, it implies that at the time of offering the treatment too little
is known about its safety. Another implication is that should sex selection be
permitted the boys born from such treatment would live with uncertainty about
their future health, beyond that normally experienced. The potential
psychological implications of this would need to be included in pre-treatment
counselling of the couples.[83]
The potential for sex selection was identified in the
Senate inquiry, with a number of scientific witnesses being of the opinion that
there was no clear justification for sex selection for male embryos if
mitochondrial donation was introduced in Australia.[84]
This issue was also considered by the NHMRC Expert Committee who reflected that
restricting mitochondrial donation to male embryos would introduce its own problematic
ethical, scientific and practical considerations.[85]
Status of
the embryo
As noted in the NHMRC community consultation report, broader
concerns about ART and the status of the embryo featured in many submissions it
received as ‘for many people, it is not possible to separate the different
technologies and issues related to embryos …’[86]
These concerns were also identified in submissions made to the Senate inquiry. Therefore,
this issue is briefly touched on, noting it does not only relate to
mitochondrial donation.
Submissions received by both the Senate inquiry and the
NHMRC raise concerns about the creation of embryos for techniques that may not
use the whole embryo and as such, part of the embryo will be disposed of (as
with PNT), or not using the embryos for reproductive purposes. A number of
submissions to the Senate inquiry reflected on the moral status of the embryos.
Some submissions expressed their view on the inherent dignity of the embryo
(and sometimes at earlier development stages, such as the zygote) which must be
respected and that embryos should not be treated as a means to an end.[87]
In contrast, some submissions received by the NHMRC
community consultation process acknowledged that embryos would be destroyed as
part of mitochondrial donation but respondents did not necessarily consider
this unethical.[88]
The rights
and wellbeing of the child
The health and wellbeing of people born from mitochondrial
donation are important factors to be considered, especially given some of the
health risks associated with the technology are still unknown.[89]
There is no way to obtain the views of a child that would
be born using mitochondrial donation. The following is an extract from the
Senate inquiry’s report on the science of mitochondrial donation and related
matters relating to the issue of whether a child would have consented to
mitochondrial donation:
People who live with a mitochondrial disease and their
children had little difficulty imagining whether a person who was born from a
mitochondrial donation technique would consent. Mary, a person living with a
mitochondrial disease, asked her children whether they thought they would have
consented to the procedure:
In the process of preparing my
submission, I talked to my children about this and I said to them: 'What do you
think?' They said, 'If we had a child with mitochondrial disease, we would love
that child we would understand what the child was going through.' But their
view was that everyone who has mitochondrial disease, their families that are
affected by it, should have this choice.
Justin, who also lives with a mitochondrial disease, told the
committee that he believes that a child who was born of the technique would
understand the choice that had been made for them:
What I have learned over the last
2½ years is that having a disability or a sickness like we've got is about
compromise. You are compromising the whole time about what you used to have in
your life that you don't have anymore. Being part of a family where the other
members of the family are disabled or are sick and is also about compromise—you
can't do the things you need to do. So I'm confident that any child, if this
technology was to go forward, born through mitochondrial donation would
understand that life is about compromises when you are in this situation. If
that means that I can't participate in recreational genetics, if that means
that a second woman gave me the slightest part of my DNA, I think that would be
a compromise I would be able to live with.[90]
[references removed]
It is also not possible to identify how a child born using
mitochondrial donation would feel about the donor (or how the donor may feel
about the child), with this potentially depending on the individuals involved
and their unique situations.[91]
In response to the NHMRC consultation, many submissions raised questions about whether
the child would be able to access information about the donor, with some people
considering it important that the child be able to do so.[92]
The privacy of the child and their family is another
consideration that is raised repeatedly. Respondents to the NHMRC community
consultation raised the need to avoid ‘medicalising’ the child but also the
need for additional information about the safety and efficacy of mitochondrial
donation.[93]
The donor
Different considerations and concerns have been raised in relation
to the donor, one of the most common ones being the idea of a child with three
parents. In the Senate inquiry, the main question this point prompted was whether
a mtDNA donor should be considered a parent.[94]
This question can be considered in two different ways:
from a genetic perspective and from a social one. With regards to genetic
contribution, several submissions and witnesses flagged the proportion of the
genetic material that the mtDNA contributes (0.1 per cent) and the role of the mtDNA.
From a social perspective, some think that the mtDNA donor can be considered
equivalent to an organ or tissue donor.[95]
For some people, organ and tissue donation is accepted and acceptable medical
practice but for others, this is not the case. One of the concerns raised about
donation is that it can impact the identity of the recipient, including, but
not limited to, the adoption of some of the traits of the donor.[96]
Whilst these concerns are not unique to mitochondrial donation, they may still
impact some people’s views on it. The Senate Committee concluded that
mitochondrial donation would not lead to a child having three parents and mtDNA
donation should be conceptualised as being similar to organ donation.[97]
In considering the donor, as is the situation with egg
donation in general, robust processes will need to be implemented to ensure
informed consent and address potential issues like coercion. In addition, harm
minimisation and respect will also need to be considered.[98]
In the NHMRC community consultation, the donor’s right to confidentiality or
even anonymity was raised as an important consideration, with submissions
suggesting donors should be able to be anonymous and other submissions stating
that donors should be identifiable.[99]
Committee
consideration
Senate Standing
Committee for Selection of Bills
At its meeting on 13 May 2021, the Senate Standing Committee
for the Selection of Bills deferred consideration of the Bill to its next
meeting.[100]
Senate
Standing Committee for the Scrutiny of Bills
The Senate Standing Committee for the Scrutiny of Bills
(the Committee) reported on this Bill on 21 April 2021.[101]
The Committee’s concerns largely arose from significant matters within the Bill
being assigned to delegated legislation.[102]
Mitochondrial
donation licence application fee
The Committee expressed concerns regarding proposed
paragraph 28H(7)(d), which provides that an application for a
mitochondrial donation licence must be accompanied by a fee, if any, prescribed
by the Regulations. The Committee questioned why the fee-making power is to be
determined by delegated legislation and why the Bill, and the Explanatory Memorandum
for the Bill, contained no information or guidance on how the fee will be calculated,
any cap on the maximum fee amount or, how to ensure that the fee will be
necessary and appropriate.[103]
The Committee considered that, at a minimum, the Bill
should include a provision stating that the fee must not be such as to amount
to taxation, noting the advice set out in paragraph 24 of the Office of
Parliamentary Counsel Drafting Direction No. 3.1.[104]
The Committee requested further advice from the Minister,
including:
- how
the amount of any fee charged will be calculated and how it will be ensured
that a fee charged to a person will be necessary and appropriate and
- whether
the Bill can be amended to provide at least high-level guidance regarding how
fees will be calculated, including, at a minimum, a provision stating that the
fee must not be such as to amount to taxation.[105]
Definition
of ‘proper consent’ and provision for withdrawal of consent
The Committee expressed concerns regarding proposed
subsection 28N(8) and proposed subsection 24(9), which provide for
the definition of 'proper consent' concerning the use of a human egg or a human
sperm for Division 4A of Part 2 and 'proper consent' concerning the use of an
excess ART embryo or a human egg for Division 4 of Part 2, respectively. Both
of these proposed subsections refer to the definition of ‘proper
consent’ as consent that is obtained under guidelines issued by the CEO of the NHMRC
under the National Health and Medical Research Council Act 1992 and
prescribed by the Regulations.[106]
The Committee also expressed concerns regarding proposed
subsection 28N(9) which provides that the Regulations may provide in
relation to the withdrawal of consent, including that consent cannot be
withdrawn in certain circumstances.[107]
The Committee questioned why significant matters, such as
provisions defining key terms as well as requirements relating to the
withdrawal of consent, are not included in the primary legislation. The
Committee acknowledged the justification, in the Explanatory Memorandum for the
Bill, for the use of delegated legislation regarding item 105 of the
Bill, but questioned why the justification did not cover proposed
subsections 28N(8) and (9) and proposed subsection 24(9).[108]
The Committee expressed general concern regarding provisions
in a Bill that allow for the incorporation of legislative provisions by
reference to other documents because such an approach:
- raises the prospect of changes being made to the law in the
absence of parliamentary scrutiny, (for example, where an external document is
incorporated as in force 'from time to time' as it is here this would mean that
any future changes to that document would operate to change the law without any
involvement from Parliament);
- can create uncertainty in the law; and
- means that those obliged to obey the law may have
inadequate access to its terms (in particular, the committee will be concerned
where relevant information, including standards, accounting principles or
industry databases, is not publicly available or is available only if a fee is
paid).[109]
The Committee requested further advice from the Minister,
including:
- why
it is considered necessary and appropriate to leave provisions defining the
scope of the term ‘proper consent’ (proposed paragraph 28N(8)(b) and proposed
subsection 24(9)) and requirements relating to the withdrawal of consent (proposed
subsection 28N(9) to delegated legislation
- whether
the Bill can be amended to include at least high-level guidance regarding these
matters on the face of the primary legislation
- why
it is considered necessary and appropriate to apply the ART Guidelines as in
force or existing from time to time (noting that this means that future changes
to the guidelines and therefore the definition of ‘proper consent’ will be
incorporated into the law without any parliamentary scrutiny) and
- whether
the Bill could be amended to provide for the meaning of 'proper consent' on the
face of the instrument or the Bill, rather than relying on the incorporation of
the ART Guidelines.[110]
Privacy
The Committee expressed concerns regarding proposed
section 28R(1)(e) and proposed section 28R(3)(d), which provides
that the Regulations may prescribe information that the holder of a clinical
trial licence or a clinical practice licence must collect for a donor, or a
child born as a result of mitochondrial donation. The Committee noted that the
Regulations (as a legislative instrument) are not subject to the full range of
parliamentary scrutiny and questioned why significant matters, such as the
collection of personal information, is not included in the primary legislation.
[111]
The Committee acknowledged the justification made in the Explanatory
Memorandum of the Bill, that delegating the collection powers to the
legislative instrument maintains administrative flexibility and is consistent
with state ART laws, but did not accept the justification to be sufficient.[112]
The Committee requested further advice from the Minister,
including:
- why
it is considered necessary and appropriate to leave the scope of sensitive
information-collection powers to delegated legislation and
- whether
the Bill can be amended to include further guidance regarding these matters on
the face of the primary legislation.[113]
The Minister’s response was received by the Committee on
10 May 2021 but had not been published at the time of writing.[114]
Policy
position of non-government parties/independents
Due to the complex nature of the Bill, the Cabinet, the Party
Room and the Opposition have agreed to allow a conscience vote, meaning members
of Parliament are not obliged to follow their party line, but can vote
according to their own moral, political, religious, or social beliefs.[115]
ALP
During the Bill’s second reading speech the Minister for
Health, Greg Hunt, stated that that the Opposition supports the Bill:
That's why, as a minister, as a father and as an individual;
with the passionate support of the Prime Minister, who is patron of the Mito
Foundation, publicly and proudly; with the support of the opposition—and Chris
Bowen has been a champion in supporting this and has passed that baton to his
successor, Mark Butler; with the support of the community; and with the support
of people such as Sarah and Joel Hood, and Catherine McGovern from the
foundation, we seek to avoid the heartache, pain and anguish of having a child
with severe mitochondrial disease.[116]
This is supported by an article reporting the then Shadow
Minister for Health, Chris Bowen, advising that Labor will support changing the
laws to allow for mitochondrial donation.[117]
In a speech in 2020, Mr Bowen stated he has had conversations with the Minister
for Health on the best way to jointly manage the process of a mature,
non-partisan debate in Parliament about the next steps needed to tackle
mitochondrial DNA disease, including mitochondrial donation.[118]
Following the second reading speech of the Bill, Labor MP Catherine
King commended the Minister for Health on the work he has done for the Bill and
acknowledged that, whilst mitochondrial donation is a difficult issue, it is
incredibly important.[119]
In September 2020, Labor MP Dr Andrew Leigh, conducted an
online and a face-to-face forum with his constituents to discuss and listen to
their views on mitochondrial donation.[120]
When asked if he already had a view on the legalisation of mitochondrial
donation, Dr Leigh stated: ‘My vote will be guided by the process, not bound.’[121]
Since the public forums, he has not offered any further views on mitochondrial
donation.
In 2019, during Private Members’ Business, Labor MP Dr Mike
Freelander spoke on the importance of legislating for mitochondrial donation.[122]
While not explicitly stating her support for the legalisation
of mitochondrial donation, during the tabling of the report prepared by the Senate
Committee inquiry in 2018, Labor Senator Louise Pratt, a member of the
Committee, reiterated her support for the report and stressed the importance of
examining the laws to see if they should be amended.[123]
The Greens
At the time of writing, only one member of the Australian
Greens had commented on their position regarding the legalisation of
mitochondrial donation. During the tabling of the report prepared by the Senate
Committee inquiry in 2018, Senator Rachel Siewert, who was Chair of the
Committee, stated:
There's no doubt in my mind that this mitochondrial donation
has a very strong potential for helping those families who are affected by
mitochondrial DNA where the disease is inherited. There's no doubt in my mind
that this has potential. But there are some things that need to occur if this
type of donation technique is to occur. There is also no doubt in my mind that
it would help affected people and those who have genetically linked children.
It would certainly help.[124]
Minor
parties and independents
At the time of writing, none of the other
minor parties or independents had formally stated a position on the Bill.
Position of
major interest groups
At the time of writing, the Mito Foundation had welcomed
the Bill but did not comment on any specific component. No other commentary on
the Bill had been identified from other interest groups.[125]
However, different groups have expressed broader views on legalising
mitochondrial donation in Australia over the course of the consultation
processes undertaken by the Senate inquiry, the NHMRC and the DoH. The
following section provides an overview of key elements raised by interested
group through the Senate inquiry. Respondents considered a number of questions
posed by the Senate Committee and provided feedback from a range of
perspectives, including responses from:
- people
with mitochondrial disease and their families
- scientists
- people
providing clinical services (for example, clinical genetics and assisted
reproduction)
- ethicists
and religious organisations.
As previously noted, these are complex and complicated
issues on which it is perhaps not possible to reach an agreed position. The
submissions included in the following section have been selected to illustrate
the range of commentary rather than nominating ‘major interest groups’ only. Not
all submissions to the Senate inquiry have been included in the following discussion,
but they are available on the Senate inquiry webpage.[126]
It should be noted that the submissions are a few years old and some positions
discussed below are also raised in the previous section on ethical and social
consideration.
Senate Community Affairs References Committee Inquiry
into: The Science of Mitochondrial Donation and Related Matters
There are
few effective treatments and no cures
In its submission to the Senate inquiry, the Mito Foundation
(the Australian Mitochondrial Disease Foundation) raised the issue that ‘there
are few effective treatments and no cures for mitochondrial disease’.[127]
The Human Genetics Society of Australasia (HGSA) noted the
importance of not over-hyping the potential of mitochondrial donation as it is
not ‘life saving’ or a ‘magic bullet’ as it does not save the life of a person
born with mitochondrial disease nor does it cure all mitochondrial DNA disease.[128]
The Mito Foundation noted this point in its supplementary submission and draws
attention to the similarities with disease prevention and health promotion
approaches.[129]
In its submission, the Australian Catholic Bishops
Conference (ACBC) raised concerns about dedicating resources to mitochondrial
donation as it does not help people who already have mitochondrial disease. The
ACBC also stated that there is a risk that this could imply people with
mitochondrial disease have lives of less worth than others.[130]
Current
reproductive options
The Mito Foundation noted in its submission that people
are choosing to remain childless to avoid the risk of their child having
mitochondrial disease. It goes on to state that mitochondrial donation is the
only option for some people with a mtDNA disorder to have children who are
genetically related to them and will not inherit mitochondrial DNA disease.[131]
The HGSA noted the emotional impact that mitochondrial
disease can have, stating the ‘clinical experience of HGSA members suggests
that mothers who have transmitted these disorders are often plagued with guilt
as there is a lack of effective treatments…’.[132]
The ACBC is of the opinion that ART is not in the best
interest of prospective parents, or their potential children, as it raises
issues affecting the dignity of all the parents and children. Instead of using
ART, ACBC identified several alternative reproductive options, noting none of
these options ‘are easy and go against the strong human desire for genetically-related
children’.[133]
Scientific
considerations
The Mito Foundation pointed out that the risks and
benefits of IVF are well known, including that it is not 100 per cent effective
or safe. It supported the introduction of mitochondrial donation in Australia
being limited to institutions and clinics with staff with demonstrated
expertise in embryology and IVF.[134]
In its submission, the HGSA stated it is ‘not aware of any
evidence to suggest that there would be significant risks to the children who
would be born following mitochondrial donation’.[135]
The Fertility Society of Australia (FSA) echoed this view, stating that ‘it
would appear that the balance of risk versus benefit in this debilitating
disease is now at a point where [mitochondrial donation] should occur’.[136]
The Biomedical Ethics Research
Group from the Murdoch Children’s Research Institute (MCRI) acknowledged the
safety concerns justifying a cautious implementation but noted that these
concerns should not legally prohibit the introduction of mitochondrial
donation.[137]
Ethical
considerations
The Mito Foundation raised concerns that failure to
introduce mitochondrial donation in Australia may result in some people
exploring fertility tourism options.[138]
In responding to certain considerations raised about egg donors, the Mito
Foundation noted that egg donation is voluntary in Australia and therefore did
not agree with concerns about the donor experience reducing the validity of
mitochondrial donation.[139]
The FSA argued that there is an ethical obligation to use a technology that
could avoid people having a disabling or fatal illness or condition.[140]
The Biomedical Ethics Research Group from the MCRI stated that there is ongoing
debate in the bioethics literature on the moral significance, if there is any,
of mitochondrial DNA and genetic connectedness.[141]
The ACBC raised several ethical concerns about
mitochondrial donation, including that PNT is a form of human cloning (due to
the transfer of nDNA) and the use of genetic material from more than two people
would threaten the rights of a child to inherit their relationship to natural
parents.[142]
The Plunkett Centre for Ethics raised similar ethical concerns about
mitochondrial donation to the ACBC and also suggested that introduction of
mitochondrial donation would open the door ‘to eugenic germ-line genetic
manipulation’.[143]
Both the ACBC and the Plunkett Centre for Ethics raised concerns about the
language ‘mitochondrial donation’ being misleading as, with some of the
techniques, the nDNA is moved rather than the mtDNA.[144]
Legal
considerations
The Australian Academy of Science recommended Australia
introduce mitochondrial donation techniques into clinical use and research,
similar to the approach taken in the UK, with due diligence of regulation and
oversight.[145]
This position is supported by the FSA and HGSA, with the HGSA strongly
advocating for a flexible and responsive governance system to minimise unintended
consequences experienced with the existing regulation.[146]
Financial
considerations
Using the Impact Statement of The Human Fertilisation and
Embryology (Mitochondrial Donation) Regulations 2015 (UK), the Mito Foundation
suggested that the introduction of mitochondrial donation could deliver a net
benefit of approximately $61 million (AUD) per year and $575 million (AUD) over
ten years.[147]
It also provided an estimate that a National Disability Insurance Scheme plan
may fund up to $120,000 per month for a child with mitochondrial disease.[148]
The Biomedical Ethics Research Group from the MCRI presented
an ethical argument on finite resources, suggesting that treating one person
with an expensive treatment can reduce the resources available for other people.
Therefore, allowing mitochondrial donation to prevent mitochondrial disease
would benefit others by increasing available capacity in the health system.[149]
The ACBC stated that given its ethical and risk related concerns, it does not
support the use of the limited resources available in both health and research for
the introduction of mitochondrial donation.[150]
Financial
implications
The Explanatory Memorandum to the Bill states that there
will be no net financial impact arising from the implementation of the Bill, and further explains:
Following the passage of the legislation, it is anticipated
that there will be some additional costs associated with establishing new
administrative processes for receiving and processing applications for and
issuing of the new mitochondrial donation licences. There will also be some
ongoing costs to Government associated with providing:
-
ongoing support for the ERLC and for ongoing compliance monitoring
related to the new licences
-
ongoing project management and support for any Commonwealth funded
clinical trial of mitochondrial donation, and
-
the establishment and maintenance of new data systems.
However, as these activities will be undertaken as an
extension of already established Government processes, the ongoing costs are
anticipated to be minimal and will be offset within the Department of Health
portfolio. In addition, whilst it is not possible to accurately predict, it is
anticipated that ten or fewer mitochondrial donation related licence
applications would likely be sought per annum.
It is also anticipated that if the clinical trial of
mitochondrial donation were to prove successful in minimising the risk of
future generations inheriting severe mitochondrial disease, this would provide
a potentially significant cost saving to the health and social welfare systems
through a reduced burden of disease and increased potential for workforce
participation. However, it not currently possible to provide estimates of these
potential savings before any outcomes of the trial have been realised.[151]
Attachment B to the Regulation Impact Statement (RIS) on
pages 86 to 94 of the Explanatory Memorandum to the Bill provides calculations
of the costs associated with the three policy proposals discussed by the RIS:
- maintain
the status quo (mitochondrial donation is not legalised in Australia)
- legalise
mitochondrial donation in Australia as a pathway to clinical use and
- support
access to overseas treatment options.[152]
Statement of Compatibility with Human Rights
As required under Part 3 of the Human Rights
(Parliamentary Scrutiny) Act 2011, the Government has assessed the Bill’s
compatibility with the human rights and freedoms recognised or declared in the
international instruments listed in section 3 of that Act. The Government
considers that the Bill is compatible.[153]
Parliamentary Joint Committee on Human Rights
The Parliamentary Joint Committee on Human Rights had no
comment in relation to the Bill.[154]
Key issues
and provisions
Overview
The use of mitochondrial donation techniques is currently
prohibited under the PHCR Act and the RIHE Act.[155]
Accordingly, the Bill amends these two Acts, and the RIHE Regulations to permit
the staged introduction of these techniques.
It is an offence under the current PHCR Act to:
- create
or develop embryos through the process of fertilisation with genetic material
from more than two people and
- make
heritable changes to the genome of a human embryo for reproductive purposes.[156]
In part, this ban does not extend to pronuclear transfer
(PNT), which is currently permitted under licence, as the technique uses two zygotes,
created with the genetic material of two people, however this technique is
prohibited from creating human embryos for reproductive purposes.[157]
This Bill proposes two significant changes which would allow, under licence, the creation of an embryo
for reproductive purposes with:
- the
genetic material of more than two people and
- changes
to its genome that would be heritable by the child’s
descendants.
Only two licences would allow for the use of these embryos
for reproductive purposes, the clinical trial licence and the clinical practice
licence. Stage 1 of mitochondrial donation will introduce the clinical trial
licence. The clinical practice licence has been identified for potential
introduction in Stage 2 of mitochondrial donation.[158]
In addition, under the RIHE Act, the woman and her
‘spouse’, if any, will be permitted to nominate only male embryos for
placement.[159]
As outlined in the background, sex selection through the use of ART is
permitted in Australia but is an option reserved for cases that would reduce
the risk of transmission of a genetic condition or disease that would severely
impact the person’s quality of life.[160]
Given the scientific and ethical considerations of sex selection for
mitochondrial donation, the Bill requires a woman/ the couple to attend
counselling before deciding if they would like to only place a male embryo.
Stage 1 of mitochondrial donation would be through a clinical
trials framework for both the licence holder and the ‘trial participant’ (that
is, the woman who is seeking to use mitochondrial donation for reproductive
purposes). Stage 2, if implemented, would consider the introduction of clinical
practice licences and any woman seeking to use mitochondrial donation for
reproductive purposes would be known as a ‘patient’ instead.
The criteria to become a trial participant (or patient)
includes evidence that the woman’s child would be at an increased risk of
developing severe mitochondrial disease. The Bill would not allow for the use
of mitochondrial donation techniques for any other reproductive purpose than to
minimise the risk of a child developing severe mitochondrial disease.[161]
Changing the
term ‘licence’ to ‘general licence’
The Bill’s introduction of the new term ‘mitochondrial
donation licence’ means that the current ‘licence’ used throughout both the RIHE
Act and the PHCR Act is no longer sufficient. ‘Licence’ is currently
defined to mean a licence issued under section 21 of the RIHE Act.[162]
In order to differentiate the ‘mitochondrial donation licences’ from the other
‘licences’ within the legislation, the Bill systematically amends the term ‘licence’
in both the RIHE Act and the PHCR Act to ‘general licence’.[163]
PHCR Act
amendments
Changes to
legalise the use of mitochondrial donation
The Bill makes significant changes to the PHCR Act by
amending sections of Part 2—Prohibited practices, allowing
for the use of mitochondrial donation techniques for reproductive purposes under
the relevant mitochondrial donation licences.
Creating human embryos for a
purpose other than achieving pregnancy
Currently, section 12 of the PHCR Act makes it an
offence to create a human embryo (by fertilising the egg with sperm outside the
body of a woman) for a purpose other than achieving pregnancy in a woman. Items
2 and 3 amend section 12 to allow the intentional creation of a
human embryo for purposes other than to achieve pregnancy in a woman, provided
the creation is authorised by the relevant mitochondrial donation licence.
This amendment has been made to allow a person to create
embryos for research and training purposes under the relevant mitochondrial
donation licences.
Creating human embryos with genetic
material from more than two people
Currently, section 13 of the PHCR Act makes it an
offence to intentionally create or develop a human embryo by the process of
fertilisation where the embryo contains genetic material provided by more than two
persons. Item 4 amends section 13 to allow the creation or development
of a human embryo that contains the genetic material of more than two persons provided
the creation is authorised by the relevant mitochondrial donation licence.
Due to the DNA contained within mitochondria, this amendment
has been made to allow the creation of embryos using DNA from three people, the
nDNA from the prospective parents and the mtDNA from the donor.
Heritable changes in the genome
Currently, section 15 of the PHCR Act makes it an
offence to make heritable changes to the genome of a human embryo. Item 5 amends
section 15 to allow heritable changes to be made to the genome of a
human embryo provided those changes are authorised under the relevant mitochondrial
donation licence.
The heritable change is in reference to the donor’s mtDNA.
Instead of inheriting the prospective mother’s mtDNA, a child born from
mitochondrial donation will inherit and, if female, has the ability to pass on
to the next generation, the donor’s mtDNA.
When prohibited embryo can be used
Currently, subsection 20(3), makes it an offence to
intentionally place an embryo in the body of a woman knowing that, or reckless
as to whether, the embryo is a prohibited embryo. The relevant prohibited
embryos for these amendments are defined in subsection 20(4) and include:
- a
human embryo created by a process other than the fertilisation of a human egg
by human sperm (paragraph (a))
- a
human embryo that contains genetic material provided by more than two persons
(paragraph (c))
- a
human embryo that contains a human cell whose genome has been altered in such a
way that the alteration is heritable by human descendants of the human whose
cell was altered (paragraph (f)).
Item 6 amends subsection 20(3) to allow for the
intentional placement of a prohibited embryo in the body of a woman, if the
embryo meets the definitions under paragraphs 20(4)(a), (c) and (f), and is
authorised under a mitochondrial donation licence.
Subsection 13.3(3) of the Criminal Code provides that
a defendant who wishes to rely on any exception, exemption, excuse,
qualification or justification provided the law creating an offence bears an
evidential burden in relation to that matter, which would require adducing or
pointing to evidence that suggests a reasonable possibility that the matter
exists. Item 7 will insert proposed subsection 20(5) into the PHCR
Act, to provide that, despite subsection 13.3(3) of the Criminal Code,
a defendant does not bear an evidential burden in relation to any matter in
subsection 20(3). This means that a person being prosecuted for an offence
under subsection 20(3) will not bear an evidential burden as to whether an
embryo is a prohibited embryo under paragraphs 20(4)(a), (c) or (f), or whether
the placement of the embryo is permitted under a mitochondrial donation licence.
The prosecution will need to prove these elements in order to establish the
offence. This reflects the approach taken in two other offences in the PHCR
Act and the RIHE Act—the offences of creating a human embryo for a
purpose other than achieving pregnancy in a woman and unauthorised use of an
excess ART embryo.[164]
Other amendments
Section 22 of the PHCR Act provides
that it is an offence to create a human embryo other than by fertilisation, or
develop such an embryo, if not authorised by licence. Section 23 provides that
it is an offence to create or develop a human embryo containing genetic material
provided by more than two people, unless authorised by licence. Item 8 changes the
nomenclature in these provisions to reflect the change from ‘licence’ to
‘general licence’ and to ensure that a person does not commit an offence under
section 22 or section 23 if their actions are authorised by a general licence,
or permitted under proposed section 28B of the RIHE Act, which
permits a person to carry out an activity as authorised by a mitochondrial
donation licence.
Definitions
added
Item 1 inserts the definitions for ‘general licence’,
‘mitochondrial donation licence’ and ‘mitochondrial donation technique’ into
the PHCR Act. These same definitions are proposed in item 10 for inclusion
in the RIHE Act (refer to Table 6 in the Appendix).
Further changes
to the PHCR Act
Item 24 of the Bill adds a note to section 4 of the PHCR
Act, which sets out the constitutional powers that support the operation of
the Act, to include reference to proposed section 28B of the RIHE Act for
activities that would be authorised by mitochondrial donation licences, relying
on the corporations power in the Constitution.
Division 2 of Part 2 of the PHCR Act (sections 22 to
23B as amended by items 8 and 28 to 30) sets out offences
relating to the creation, development or use of specified embryos, unless
authorised by a general licence or a mitochondrial donation licence. Sections
12, 13, 15 and 20 (as amended by items 2 to 7 of the Bill) set
out offences relating to the creation, development, use or placement of specified
embryos, unless authorised by a mitochondrial donation licence. These offences
will be referred to as licence offences (proposed subsection
23BA(2) at item 31). Proposed subsection 23BA(1) clarifies
that a person cannot be held criminally responsible for a licence offence
in respect of particular conduct authorised by a provision of a general licence
or a mitochondrial donation licence if that licence or provision was invalid
for some reason and the person did not know, and could not reasonably be expected
to have known, about the invalidity.
RIHE Act
amendments
Definitions
added
Item 9 inserts the definitions outlined in Table 6
in the Appendix, into section 7 of the RIHE Act.
Item 10 inserts the definitions outlined in Table 6
in the Appendix, into section 8 of the RIHE Act.
Changes to
the offence provisions of the RIHE Act
Item 11 expands subsection 10(1) of the RIHE Act,
which is an offence provision, prohibiting the use of excess ART embryos unless
permitted under licence or if certain exceptions apply. Proposed paragraphs
10(1)(a) and (aa) allow use of excess ART embryos in accordance with
a mitochondrial donation licence and amends the language of ‘licence’ to ‘general
licence’.
Currently, paragraph 10(2)(e) provides for the use of an
excess ART embryo in accredited ART centres for reproductive purposes in a
woman other than the woman for whom the embryo was created. Item 12
amends this paragraph so that the use of an excess embryo in this way will only
be permitted where that embryo was not created using a mitochondrial donation
technique as authorised by a mitochondrial donation licence.
Section 10A provides that it is an offence to use specified
types of embryos, where that use is not authorised by a licence. (The specified
embryos are embryos that were created by a process other than the fertilisation
of a human egg by a human sperm; that contain genetic material provided by more
than two people; that were created using precursor cells taken from a human
embryo or a human fetus; or which are hybrid embryos.) Item
13 amends paragraph 10A(c) to update the term ‘licence’ to ‘general
licence’. It will also ensure that the offence does not apply to use of an
embryo created by a process other than the fertilisation of a human egg by a
human sperm, or that contains genetic material provided by more than two people,
if that use is authorised by a mitochondrial donation licence.
Section 10B provides that it is an offence to undertake
research or training involving the fertilisation of a human egg by a human
sperm up to, but not including, the first mitotic division, outside the body of
a woman, unless authorised under licence. Item 14 amends section 10B to
update the term ‘licence’ to ‘general licence’. It will also ensure that the
offence does not apply to such research and training authorised by a
mitochondrial donation licence.
Section 11 provides that it is an offence to intentionally
use, outside the body of a woman, a human embryo that is not an excess ART
embryo, where the use is not carried out by an accredited ART centre for a
purpose relating to the ART treatment of a woman, and the person knows or is
reckless as to that fact. Item 15 amends section 11, so that the offence
does not apply where the use of the embryo is permitted under a mitochondrial
donation licence.
Item 16 inserts proposed section 11A, making
it an offence for people to use material created under a mitochondrial donation
licence for any other activities than the ones authorised by the licence.
Mitochondrial donation licences
The RIHE Act regulates research involving human
embryos, providing a regulatory framework for a licensing system that is
overseen by the NHMRC Licensing Committee.[165]
Item 17 creates a new Division 4A in Part 2 of the RIHE Act
and contains most of the provisions that regulate mitochondrial donation
licences and licensing, with many similarities to the existing licensing
process. Proposed Division 4A contains proposed sections 28A to 28X.
Kinds of mitochondrial donation
licences and what they authorise
Proposed section 28A identifies five types of mitochondrial
donation licence:
- pre-clinical
research and training licence
- clinical
trial research and training licence
- clinical
trial licence
- clinical
practice research and training licence
- clinical
practice licence.
The Bill provides for the first three of these licences to
be issued, as part of the Stage 1 implementation of mitochondrial donation,
with the Australian Government intending to authorise one clinic to undertake a
Commonwealth funded clinical trial.[166]
Proposed subsection 28B(1) permits preclinical research and
training, clinical trial research and training and clinical trial licence
holders to use mitochondrial donation techniques if their licence is in force
and the licence holder is a constitutional corporation (that is, a trading,
foreign or financial corporation within the meaning of paragraph 51(xx) of the Constitution—see
item 9). Proposed subsection 28B(2) permits preclinical research
and training, clinical trial research and training, and clinical trial licence
holders to carry out activities under their licence despite state or territory
law. Further amendments would be required to the RIHE Act and the RIHE
Regulations to permit mitochondrial donation techniques for clinical practice
as well as changes to state or territory legislation to permit the use of these
techniques in a particular state or territory (proposed subsection 28B(3)).[167]
The purpose of a mitochondrial
donation licence
The purpose of a preclinical research and training licence
is to:
- develop
the technique specified in the licence for potential
future use in a clinical setting to minimise the risk of
a child inheriting mtDNA that could predispose them to mitochondrial DNA disease
(proposed paragraph 28C(1)(a))
- better
understand the technique (proposed paragraph 28C(1)(b))
- build
staff expertise (proposed paragraph 28C(1)(c)).
The purpose of a clinical trial research and
training licence is to:
- develop
protocols for using the technique specified in the licence safely and
effectively in a clinical trial setting, to minimise the risk of a child inheriting
mtDNA that could predispose them to mitochondrial DNA disease (proposed
paragraph 28D(1)(a))
- ensure
the competency of the embryologist/s identified on the licence (proposed
paragraph 28D(1)(b)) and
- ensure
the centre is sufficiently set up for using the technique in a clinical trial (proposed
paragraph 28D(1)(c)).
The purpose of a clinical trial licence is
to determine whether the technique specified in the licence is safe and
effective for potential use in a clinical practice setting to create a human
embryo and place that embryo in a trial participant to achieve a pregnancy (proposed
subsection 28E(1)).
The purpose of the clinical practice research and
training licence mirrors that of a clinical trial research and practice
licence, but with the intention of preparing for use in the clinical practice
setting instead (proposed subsection 28F(1)).
The purpose of the clinical practice licence
is to create a human embryo using the technique specified in the licence with
the intention to place the embryo in the patient to achieve a pregnancy (proposed
subsection 28G(1)).
Activities authorised under
mitochondrial donation licences
With the exception of the pre-clinical research and
training licence, licence holders will need to undertake the activities approved
in their licence in an accredited ART centre.[168]
Table 2 provides an overview of the activities permitted under the five
different licences.
Table 2: activities authorised under mitochondrial
donation licences
|
Activity
|
Pre-clinical research
and training licence
|
Clinical trial research
and training licence
|
Clinical trial licence
|
Clinical practice
research and training licence
|
Clinical practice
licence
|
| Proposed subsection |
28C(2) |
28D(2) |
28E(2) |
28F(2) |
28G(2) |
| Creation of human embryos
other than by fertilisation |
√ |
√ |
√ |
√ |
√ |
| Creation of human embryos
with genetic material from more than two people |
√ |
√ |
√ |
√ |
√ |
| Creation of human embryos
through fertilisation outside of the body of a woman |
√ |
√ |
√ |
√ |
√ |
| Creation of human embryos
with heritable changes to its genome |
× |
× |
√ |
× |
√ |
| Research and training
involving fertilisation |
√ |
√ |
× |
√ |
x |
| Use of material created,
developed or produced under a licence (other than excess ART embryos) |
√ |
√ |
√ |
√ |
√ |
| Placement in a woman of an embryo
created by a mitochondrial donation technique for reproductive purposes |
× |
× |
√ |
× |
√ |
| Development of an embryo
for more than 14 days |
× |
× |
× |
× |
× |
Source:
Mitochondrial Donation Law Reform
(Maeve’s Law) Bill 2021; Explanatory Memorandum, Mitochondrial Donation Law Reform
(Maeve's Law) Bill 2021, p. 65.
Applying for
a mitochondrial donation licence
A person will need to apply to the NHMRC Licensing
Committee for a mitochondrial donation licence, specifying the technique and
activities they wish to undertake (proposed subsection 28H(1)). For some
licence types, there are prerequisites the applicant will need to meet:
- only
a person who has held a clinical trial research and training licence for the specific
technique can apply for a clinical trial licence (proposed subsection 28H(3))
- only
a person who has held a clinical practice research and
training licence for the specific technique can apply for a clinical
practice licence (proposed subsection 28H(4)).[169]
The information provided in the Explanatory Memorandum is
slightly different, stating: ‘in order to conduct a clinical trial in a
mitochondrial donation technique, both a clinical trial research and training
licence and a clinical trial licence will be needed’.[170]
The reasoning provided for this dual requirement is that the clinical trial
research and training licence would allow for the preparation of the clinical
trial, whilst the clinical trial licence would allow for use of the technique
for reproductive purposes.[171]
Only a constitutional corporation may apply for a pre-clinical
research and training licence, a clinical trial research and training licence,
or a clinical trial licence (proposed subsection 28H(2)).
Each application will be required to:
- nominate
at least one embryologist, who would be authorised to use the technique (proposed
subsection 28H(5))
- apply
for only one type of licence (proposed paragraph 28H(6)(a))
- apply
for only one mitochondrial donation technique (proposed paragraph 28H(6)(b)).
Proposed subsection 28H(7) outlines the information
the applicant will need to provide, in an approved form, as determined by the
NHMRC Licensing Committee:
- identify
the type of licence applied for
- specify
the technique
- meet
the requirements specified in the Regulations (if any)
- meet
the other requirements identified by the NHMRC Licensing Committee
- be
accompanied by the fee prescribed by the Regulations (if any).[172]
Determination of application by the
NHMRC Licensing Committee
Proposed section 28J specifies the criteria the NHMRC
Licensing Committee will need to consider before deciding to issue a licence.
The NHMRC Licensing Committee would need to be satisfied:
- the
appropriate protocols are in place to obtain proper consent (defined below) and
to ensure compliance with any restrictions to the consent (proposed paragraph
28J(2)(a))
- the
activities in the application have been assessed and approved by a Human Research
Ethics Committee (HREC), which is compliant with the National
Statement on Ethical Conduct in Human Research (National
Statement) (proposed paragraph 28J(2)(b)).[173]
The NHMRC Licensing Committee will need to consider the
following information in its deliberations on whether to issue a licence:
- restricting
the number of excess ART embryos, other embryos, human eggs, or zygotes to what
is likely to be required to achieve the goals of the proposed activities in the
application (proposed paragraph 28J(3)(a))
- any
relevant guidelines issued by the NHMRC (proposed paragraph 28J(3)(b))
- the
HREC assessment (proposed paragraph 28J(3)(c))
- whether
the applicant has complied with conditions of other mitochondrial donation
licences, as relevant (proposed paragraph 28J(3)(d)).
Proposed subsection 28(J)(4) will allow the NHMRC
Licensing Committee to seek, and have regard to, the advice of appropriate
experts.
Expansion of considerations in
issuing a clinical trial licence
Proposed subsection 28(J)(5) expands on the
considerations the NHMRC Licensing Committee would need to be satisfied of
before issuing a clinical trial licence or a clinical practice licence:
- the
applicant has appropriate protocols in place for the technique to be safe and
effective in minimising the risk of a child inheriting mitochondria that could
predispose them to mitochondrial disease
- each
embryologist identified in the application has:
- consented
in writing to be nominated
- demonstrated
technical competence in the use of the nominated technique
- understands
their obligations under the Act
- the
applicant’s facilities, equipment and processes are fit for purpose
- the
staff who would be directly involved in the clinical trial or clinical practice
have the appropriate qualifications, training and competencies
- the
applicant is likely to be able to comply with their obligations under proposed section
28R (information about donors and children, discussed below)
- the
applicant has protocols in place to ensure any donors would be aware that any
children resulting from a pregnancy that used their egg would be able to obtain
information about them through the Mitochondrial Donation Donor Register
- the
applicant has protocols to ensure trial participants or patients are fully
informed about:
- the
risks involving the mitochondrial donation techniques
- alternatives
to mitochondrial donation.[175]
In addition, the regulations may specify further requirements
for the NHMRC Licensing Committee and/or the need for each embryologist to
demonstrate their technical competence with the nominated technique.
Notification of decision and
matters specified in the licence
Consistent with the existing notification requirements for (general)
licences in section 22 of the RIHE Act, proposed section 28K outlines
requirements for the NHMRC Licensing Committee to notify, and provide a copy of
the mitochondrial donation licence, to:
- the
applicant
- the
HREC
- the
relevant state/territory body of the jurisdiction in which the activities will
occur.
Proposed section 28L outlines three matters that
must be specified in the licence:
- the
approved technique
- the
activities that have been authorised
- the
name of each embryologist.
In line with existing requirements in section 23 of the RIHE
Act for (general) licences, a mitochondrial donation licence would come
into force either on the date of issue or the date specified on the licence and
remain in force until the date specified on the licence, unless it is
suspended, revoked or surrendered before that day (proposed section 28M).
Conditions of mitochondrial
donation licences
Prior to a human egg or a human sperm being used, the
licence holder will need to ensure:
- proper
consent has been obtained (proposed paragraph 28N(1)(a))
- the
NHMRC Licensing Committee has been notified in writing that this consent has
been obtained, including any restrictions the consent is subject to (proposed
paragraph 28N(1)(b)). This notification must not include identifiable
information for the responsible person (biological donor) (proposed
subsection 28N(2)).
If restrictions were placed on the responsible person’s
consent, then the licence would be subject to those conditions (proposed subsection 28N(3)).
Proposed subsection 28N(5) is based on subsections
24(4) and (5), which apply to (general) licences, and outlines other conditions
that may be specified in the licence, including:
- the
persons authorised by the licence, including the embryologists, to carry out the
authorised activities
- the
number of human eggs that can be used under the licence, or the number of
embryos or zygotes that can be created or used under the licence[176]
- reporting
requirements
- monitoring
- the
licence holder will need to provide information about the licence to the
embryologists and other persons authorised by the licence
- disposing
of material produced by the technique.
The licence conditions apply to the licence holder, each
embryologist named on the licence and each person who carries out activities
authorised by the licence (proposed subsections 28N(6) and (7)).
Proposed subsection 28N(8) gives two definitions
for proposed Division 4A of Part 2 of the RIHE Act:
- Proper
consent, in relation to the use of a human egg or sperm, means consent
is obtained in line with the NHMRC guidelines that are prescribed under Regulations
and any other requirements specified in the regulations. Proposed section 7J
of the RIHE Regulations (at item 20 of the Bill), prescribes the ART
Guidelines.[177]
Other requirements prescribed in the Regulations may include (but are not
limited to) how to withdraw consent and circumstances in which consent cannot
be withdrawn.
- Responsible
person, in relation to the use of a human egg or human sperm, refers to
the biological donor of that egg or sperm.
Additional conditions for clinical
trial and clinical practice licences
Additional conditions apply to clinical trial licences and
clinical practice licences which require approval from the NHMRC Licensing
Committee before the creation or placement of an embryo (proposed
section 28P). The NHMRC Licensing Committee must be satisfied of the
following before granting this approval:
- there
is a particular risk of the woman’s offspring inheriting mtDNA from the woman that
would predispose them to mitochondrial disease
- there
is a significant risk that the mitochondrial disease that would develop in
those offspring would result in serious illness or other serious medical conditions
- that
other available techniques could potentially be used to minimise the risks
would be inappropriate or unlikely to succeed
- that
the woman and her spouse (if any) have attended counselling and been fully
informed of:
- the
risks involved in using mitochondrial donation techniques and
- alternatives
to using mitochondrial donation techniques
- that
the woman has given written consent to the making of the application and
- other
matters as specified in the regulations (proposed
subsection 28P(4)).
In addition, the NHMRC Licensing Committee must consider:
- the
clinical basis of the risk to the woman’s offspring of mitochondrial DNA disease
- the
inheritance pattern in the woman’s family
- the
likely clinical manifestation of the disease in the woman’s offspring (proposed subsection 28P(5)).
If approval is granted, it comes into force when it is
granted and ceases at the earlier of the following:
- five
years following approval
- when
a child is born alive as a result of placement following mitochondrial donation
(proposed subsection 28P(8)).
In addition, following counselling, the woman and her
spouse can request only male embryos be used for placement (proposed
subsection 28Q(1)).[178]
Ongoing requirements for mitochondrial
donation licence holders
Proposed section 28R outlines the requirements for clinical
trial licence holders and clinical practice licence holders to record specific
information about the donor and children born following mitochondrial donation.
The clinical trial or clinical practice licence holder
must collect specific information about the donor:
- their
full name
- their
residential address at the time they provided proper consent
- their
date and place of birth
- any
additional information provided by the donor for the purposes of inclusion on
the Mitochondrial Donation Donor Register
- any
other information required under the Regulations.
A woman becomes known as a donor when a zygote is created
using her mitochondria and the nDNA from another woman.
The person who is or was the licence holder must endeavour
to collect the following information for each child born alive following
mitochondrial donation:
- their
full name
- their
sex
- their
date of birth
- any
other information prescribed by the Regulations.
The records on the donor and the child need to be retained
for the period prescribed in the regulations. Proposed section 7K of the
RIHE Regulations (at item 30 of the Bill) provides that the records must
be retained for 25 years after their creation.
If the person who is or was the licence holder becomes
aware that a child has been born alive, they will need to notify the Secretary
and the NHMRC Licensing Committee. In addition, they will need to notify the
Secretary of the donor and child’s details.
It is an offence to intentionally engage in conduct
knowing that, or reckless as to whether, the conduct breaches the above
requirements. The maximum penalty for this offence is imprisonment for two
years.
Adverse events - ongoing monitoring
and reporting requirements for clinical trial and
clinical practice licence holders
Proposed section 28S outlines the monitoring and
reporting requirements of the person who is or was the licence holder of a
clinical trial licence or clinical practice licence, who will need to have
protocols in place to:
- monitor
the pregnancy and childbirth
- monitor
the ongoing health and development of the child
- seek
the ongoing engagement of trial participants (and patients) who achieve
pregnancy and children to engage with this monitoring
- notify
the below entities of any ‘adverse events’:
- the
NHMRC Licensing Committee
- the
Secretary
- any
additional people as prescribed in the Regulations.
The Regulations require notification of an adverse event to be
made in a form approved by the CEO of the NHMRC and to contain any information
required by the form (proposed section 7L of the RIHE Regulations, at item 20 of the Bill). IVF conceived babies
have a recorded higher incidence of spontaneous premature birth.[179]
To identify if there are any additional risks associated with the use of
mitochondrial donation techniques, this form will likely require that in the
case of premature birth, information be provided on how premature the birth
was.[180]
An ‘adverse event’ for a trial participant or patient
or a child of a trial participant is defined in proposed section 7M of
the RIHE Regulations, at item 20 of the Bill, as follows:
- for
a trial participant or a patient:
- a
failed embryo development
- a
miscarriage
- a
premature birth of a child or
- a
child born with a birth defect, a genetic abnormality or a diagnosis at birth
of mitochondrial disease.
- for
a child of a trial participant:
- a
diagnosis at any time of mitochondrial disease.
In the instance of a notification of an adverse event, the
person providing the advice must not include any information that could be used
to discover the identity of the trial participant/patient and/or the child (proposed
subsection 28S(5)).
It is an offence to intentionally engage in conduct knowing
that, or reckless as to whether, the conduct breaches the above requirements.
The maximum penalty for this offence is imprisonment for two years (proposed
subsection 28S(7)).
The Regulations may prescribe record-keeping requirements
that may include penalties, not exceeding 50 penalty units, for offences
against the regulations. The value of a penalty unit is currently $222.[181]
Variation, suspension, revocation
and surrender of a licence
The NHMRC Licensing Committee can, by providing notice in
writing to the licence holder, vary the licence if the Committee believes, on
reasonable grounds, that it is necessary or desirable. This can be done in
response to an application from the licence holder or on the Committee’s initiative
(proposed section 28U).
The Committee may suspend or revoke a licence if it
believes on reasonable grounds that a condition of the licence has been
breached. If the holder of a licence is convicted of an offence under the RIHE
Act or the Regulations, or the PHCR Act, the NHMRC Licensing
Committee must revoke the licence/s held by the licence holder. If the licence
holder for any of the pre-clinical or clinical trial licences ceases to be a
constitutional corporation, the NHMRC Licensing Committee is taken to have
revoked the licence at that time (proposed section 28V).
The licence holder can surrender the licence by giving the
NHMRC Licensing Committee written notice (proposed section 28W).
If the NHMRC Licensing Committee does vary, suspend or
revoke a licence it will need to notify the licence holder, the HREC and the
relevant state or territory body. In addition, if a licence is surrendered, the
Committee will need to inform the relevant HREC and state or territory body (proposed
section 28X).
Mitochondrial Donation Donor Register
Item 18 of the Bill introduces proposed section
29A into the RIHE Act, which details the requirement for a Mitochondrial
Donation Donor Register (the Register) to be established. The purpose of the Register
is to allow any person who is over the age of 18 and was born as a result of
the use of mitochondrial donation to apply for, and be provided with, identifiable
information regarding their mitochondrial donor.
Information kept
in the register
The register must be updated and kept by the Secretary of
the DoH (proposed subsection 29A(1)) or a delegated SES employee or
acting SES employee in the Department (proposed subsection 29A(8)). The Register
must include the following information, outlined in proposed paragraph
28R(5)(b):
- the
donor’s full name
- the
donor’s residential address at the time that the donor gave proper consent
- the
donor’s date and place of birth
- any
other information the donor has provided to a licence holder at the time that
the donor gave proper consent
- the
name, sex and date of birth of each child born alive as a result of a pregnancy
achieved using a mitochondrial donation technique
- any
other information prescribed by the Regulations.
Who can
access register information?
Information in the Register cannot be made publicly
available (proposed subsection 29A(3)). An application for access to
information in the Register may be made by:
- a
person born as the result of mitochondrial donation, who is over the age of 18
(proposed subsection 29A(4))
- a
donor (proposed subsection 29A(5)).
People over the age of 18 who were born as the result of
mitochondrial donation can apply for identifiable information about their
donor. Donors are only able to apply for information from the register about
themselves, in relation to the use of a mitochondrial donation technique. Paragraph
167 of the Explanatory Memorandum of the Bill states that a donor will be able
to discover whether a child has been born from their donation.[182]
However, proposed subsection 29A(5) provides that a donor may
only apply to view the information in the register about themselves, as
described in proposed subsection 28R(1). This information does not
include whether a child has been born from their donation. Proposed paragraph
28R(1)(e), which requires information prescribed by the Regulations to be
recorded about the donor, specifies that the information must be ‘about the
donor’. When applied for by the people outlined above, the information must be
provided (proposed subsection 29A(6)).
It is a criminal offence for a person to disclose
information on the Register (where they have that information by performing
functions associated with the Register) for reasons other than those outlined
in proposed subsections 29A(4) and (5), or in accordance with a
court order. The maximum penalty for committing such an offence is two years
imprisonment (proposed subsection 29A(7)).
The privacy of the donor
The donor’s rights to confidentiality or anonymity have
been raised during community consultation as an important consideration. The function
of the register to allow people born of mitochondrial donation techniques who
are aged over 18 to access personal information about their donor means they
would be able to contact their donor if they so desire.
This approach differs from the UK’s model for mitochondrial
donors.[183]
In the UK, children born of mitochondrial donation who are over the age of 16
can access the following non-identifying information about their donor:
- information
on the donor’s personal and family medical history
- a
personal description (if provided), and
- any
additional information the donor has agreed to share with the child.[184]
During the community consultations conducted by the NHRMC,
arguments were made both for and against the disclosure of a donor’s personal
information. Proponents and opponents appear to agree that the donor’s medical and
genetic information should be available for health purposes.[185]
Register management
The RIHE Regulations may provide for the following aspects
of the Register (proposed subsection 29A(10)):
- other
information to be kept on the Register
- correcting
and updating information
- keeping
and maintenance
- verification
of the information in an application for the disclosure of information on the Register.
The Bill’s amendments to the Regulations do not include
any provisions for the details of the Register outlined in proposed
subsection 29A(10). Given a child born of mitochondrial donation must wait
18 years before applying for information from the Register, the consistent
management and upkeep of the information will be important to ensure that
correct information is supplied to the applicant.
Mitochondrial
donation techniques
Item 19 inserts the definitions of the five
mitochondrial donation techniques into section 5 of the RIHE Regulations, which
sets out definitions.
Item 20 inserts proposed Division 2–Provisions
relating to mitochondrial donation licence into Part 2 of the Regulations.[186]
Proposed section 7A prescribes
five mitochondrial donation techniques for the purposes of the proposed definition
in section 8 of the RIHE Act, inserted by item 10 of the Bill:
- maternal
spindle transfer (MST)
- pronuclear
transfer (PNT)
- germinal
vesicle transfer (GVT)
- first
polar body transfer (PBT)
- second
polar body transfer (PBT).
Permitted
techniques for mitochondrial donation licences
Proposed section 7B defines the permitted
techniques for the mitochondrial donation licences that will be available under
Stage 1, as outlined below in Table 3.
Table 3: permitted
techniques for the mitochondrial donation licences.
| Mitochondrial donation licences |
Permitted techniques |
| Pre-clinical research and training licence |
MST, PNT, GVT and PBT |
| A clinical trial research and training licence or a clinical trial licence |
MST and PNT |
Source: Mitochondrial Donation Law Reform
(Maeve’s Law) Bill 2021.
Mitochondrial
donation techniques definitions
Proposed sections 7C, 7D, 7E, 7F
and 7G insert the definitions for MST, PNT, GVT, PBT (1st and 2nd)
respectively.
Table 4 below provides a simple definition and explanation
of how the five techniques work.[187]
Table 4:
mitochondrial donation techniques definitions
| Term |
Definition |
Proposed sections |
| GVT |
A technique that involves removing the germinal vesicle
(which contains the mother’s nDNA) from the prospective mother’s immature egg
cell. The germinal vesicle is then placed into a donor egg from which the
donor’s germinal vesicle (and therefore the donor’s nDNA) has been removed.
Once the germinal vesicle has been transferred to the donated egg, and the
egg matures, it is fertilised by the father’s sperm to create a zygote. |
7E |
| PBT |
A technique that involves removing the prospective
mother's polar body (which contains the mother’s nDNA) and fusing it to a donor
egg that has had the donor’s nDNA removed. This reconstituted egg is either
then fertilised by the prospective father’s sperm to form a zygote (1st PBT)
or has already been fertilised by the prospective father’s sperm before the
transfer (2nd PBT). |
7F and 7G |
| PNT |
A technique that involves fertilisation of both the
prospective mother and the donor's egg cell with the prospective father’s
sperm to create two zygotes (a maternal zygote and a donor zygote). The
pronuclei are then removed from the maternal zygote and transferred to the
donor zygote, which has had its own pronuclei (and therefore the donor’s
nDNA) removed but which retains its own intact mitochondria. |
7D |
| MST |
A technique that involves removing the maternal spindle
from an egg cell of the prospective mother. The spindle is then placed into a
donor egg from which the donor’s maternal spindle (and therefore the donor’s
nDNA) has been removed. Once the maternal spindle has been transferred to the
donated egg, this egg is fertilised by the father’s sperm to create a zygote. |
7C |
Source: Mitochondrial Donation Law Reform (Maeve’s Law) Bill
2021.
Further
changes to the RIHE Act
Item 33 adds a note to section 4 of the RIHE Act,
which sets out the constitutional powers that support the operation of the Act,
to include reference to proposed section 28B for activities that would be
authorised by mitochondrial donation licences, relying on the corporations
power in the Constitution.
Section 20 (as amended by items 57 to 60 of
the Bill) allows a person to apply to the NHMRC Licensing Committee for a
general licence authorising the creation of human embryos, the use of excess
embryos, and research and training involving fertilisation of a human egg. Item
60 clarifies that the NHMRC Licensing Committee is not permitted to
authorise a mitochondrial donation technique, or the use of material created,
developed or produced from a mitochondrial donation technique, under a general
licence.
Item 71 inserts definitions of proper consent
and responsible person that apply to Division 4 of Part 2 of the
RIHE Act (general licences). (See Table 6 for additional information).
Section 29 of the RIHE Act (as amended by items
83 to 88 of the Bill), requires the NHMRC Licensing Committee to
make certain information on general licences and mitochondrial donation
licences publicly available on a database. Such information includes the name
of the person to whom a licence is issued, licence conditions and, for a
general licence, the number of ART embryos or human eggs authorised to be used
under the licence, and the number of other embryos authorised to be created or
used under the licence. Items 85 and 88 amend section 29 to
insert two additional information requirements for mitochondrial donation
licences. These amendments require the database to include, for each
mitochondrial donation licence:
- a
short statement on the use of excess ART embryos, human eggs and other embryos
authorised under the licence and
- the
number of excess ART embryos or human eggs authorised to be used under the
licence, and the number of other embryos or zygotes authorised to be created or
used under the licence.
Section 32 of the RIHE Act provides for certain
decisions of the NHMRC Licensing Committee to be reviewed by the Administrative
Appeals Tribunal (AAT). Items 95 to 100 amend section 32 to
provide that the following decisions of the NHMRC Licensing Committee in
relation to mitochondrial donation licences are also reviewable by the AAT:
- a
decision under proposed section 28J of the RIHE Act (at item
17) not to issue a mitochondrial donation licence
- a
decision under proposed section 28M (at item 17) in relation to
the period of a mitochondrial donation licence
- a
decision under proposed subsection 28N (at item 17) to specify a
condition in a mitochondrial donation licence
- a
decision under proposed section 28U (at item 17) to vary or
refuse to vary a mitochondrial donation licence
- a
decision under proposed section 28V (at item 17) to suspend or
revoke a mitochondrial donation licence
- a
decision under proposed subsection 28P(3) (at item 17) not to
grant an approval to create a human embryo for a trial participant or patient
using a mitochondrial donation technique or place such an embryo in the body of
the trial participant or patient.
Item 103 repeals Division 1 and 2 of Part 5 of the RIHE
Act and substitutes them with proposed Division 1—Arrangements relating
to clinical trials of mitochondrial donation techniques and proposed
Division 2—Other miscellaneous matters.
Proposed
Division 1—Arrangements relating to clinical trials for mitochondrial donation
techniques
Broadly speaking proposed Division 1 of Part 5 of
the RIHE Act purpose is to provide Commonwealth legislative authority
for expenditure on a clinical trial of a mitochondrial donation technique, as
well as the associated research and training.
Proposed subsection 46(1) provides the Commonwealth
legislative authority to enter into a contract (or agreement, deed or other
arrangement) with a constitutional corporation regarding conducting a clinical
trial under a clinical trial licence, and the associated activities. It will also
allow the Commonwealth to pay a constitutional corporation for that purpose. This
legislative authority can be exercised by the Minister or by the DoH Secretary
(proposed subsection 46(2)). The Minister and the Secretary can delegate
their powers under proposed section 46B, discussed further below.
Terms and conditions relating to clinical
trial arrangements
Proposed section 46A requires that the terms and
conditions relating to clinical trial matters must:
- be
set out in a written agreement between the Commonwealth (or by the Minister or
the Secretary on behalf of the Commonwealth) and the corporation
- be
complied with by the corporation and
- outline
any circumstances in which the corporation must repay amounts to the
Commonwealth.
Minister or Secretary may delegate
powers in relation to arrangements
Proposed section 46B details the powers of
delegation for both the Minister and the Secretary. The Minister and the
Secretary may delegate their powers under proposed section 46 or 46A
to an SES or acting SES employee (proposed subsections 46B(1) and (3)).
The delegate must comply with the directions of the Minister or the Secretary (proposed
subsections 46B(2) and (4)).
Proposed section 46D ensures that proposed
Division 1 of Part 5 does not limit the executive powers of the
Commonwealth (see section 61 of the Constitution).
Proposed
Division 2—Other miscellaneous matters
Proposed section 47 provides clarification that for
the purposes of the Gene
Technology Act 2000, mitochondrial donation techniques are not
considered to be gene technology when authorised under a mitochondrial donation
licence. This inclusion means that any child born following the use of a
mitochondrial donation technique would not be meet the definition of a
‘genetically modified organism’.
Proposed subsection 47A provides immunity for the
Commonwealth and a ‘protected person’ (defined in the table below) from civil
actions, suits and proceedings in respect of loss, damage or injury of any kind
suffered by another person as a result of the actions and omissions outlined
below in Table 5.
Table 5:
Immunity from civil actions relating to mitochondrial donation licences
| Protected persons |
Protected matters |
|
Any of the following persons:
- the
Minister
- the
Secretary
- a
person to whom powers or functions are delegated under proposed
subsections 29A(8)[188]
- an
inspector
- an
officer or employee of the Department
- a
member of the NHMRC Licensing Committee
- the CEO
or an employee of the NHMRC
- a
member of a HREC.
|
The performance or purported performance, or the exercise
or purported exercise, of the person’s functions, duties or powers under the
following in so far as they relate to mitochondrial donation licences:
- the RIHE
Act or a legislative instrument made under it
- the PHCR
Act or a legislative instrument made under it.
|
|
A person who the NHMRC Licensing Committee requests, or
purportedly requests, to provide advice as mentioned in proposed
subsection 28J(4).[189]
|
The provision, or purported provision, by the person of
advice in response to such a request.
|
|
A person who gives, or purportedly gives, information to
the Secretary under proposed paragraph 28R(5)(b).[190]
|
The giving, or purported giving, of the information by the
person.
|
Source: Mitochondrial Donation Law Reform
(Maeve’s Law) Bill 2021.
There are known risks associated with mitochondrial
donation, and because of the newness of the mitochondrial donation techniques for
human reproductive purposes, there is potential for yet unknown consequences to
emerge.[191]
Due to these known risks and the potential for yet unidentified risks, the
protection afforded to the Commonwealth and a ‘protected person’ under proposed
subsection 47A allows these persons to undertake their administrative tasks
without fear of civil liability for adverse events that might arise from the
use of a mitochondrial donation technique.[192]
Proposed subsection 47A(2) makes the exception that
the above protection does not apply to an act or omission in bad faith.
Proposed subsection 47A(4) ensures that the
protection provided above is subject to section 40 of the RIHE Act, which
provides for compensation for damage to equipment or other facilities in
certain circumstances.
Changes to
the Therapeutic Goods (Excluded Goods) Determination 2018
Subsection 7AA(2) of the Therapeutic Goods
Act 1989 (TG Act) allows the Minister, by legislative
instrument, to determine that specified goods when used, advertised, or
presented for supply in a way specified in the determination, are excluded
goods for the purposes of the TG Act. Excluded goods are not
therapeutic goods and are therefore not subject to the requirements relating to
import, export, manufacture or supply under the TG Act. Schedule 2 of the
Therapeutic Goods
(Excluded Goods) Determination 2018 sets out certain goods and the manner
in which they must be used, advertised or presented for supply in order to be excluded
goods.
Item 116 amends Schedule 2 of the Therapeutic Goods
(Excluded Goods) Determination 2018 to provide that human eggs and human
sperm are excluded goods when used in activities authorised by a mitochondrial
donation licence. This inclusion means that when these goods are used in the
authorised way, they are exempt from the operation of the TG Act.
Seven-year
review of the new arrangements
Current sections 25 and 25A of the PHCR Act and
sections 47 and 47A of the RIHE Act provide for two reviews of those
Acts, to be undertaken concurrently. As both these reviews have taken place,
these sections are superfluous. Items 32 and 103 repeal those
sections and replace them with proposed section 25 of the PHCR Act
and proposed section 47B of the RIHE Act, respectively. The
proposed provisions will require the Minister to arrange an independent review
of the operation of the PHCR Act and RIHE Act, in so far as they
relate to the use of mitochondrial donation techniques, to be undertaken as
soon as possible after the end of:
- the
period of seven years starting on the commencement of Schedule 1 to the Bill and
- each
subsequent seven-year period.
The people undertaking the Review are chosen by the
Minister, with agreement from each state and territory (proposed subsection
25(2) of the PHCR Act and proposed paragraph 47B(2)(a) of the
RIHE Act). The reviews must be undertaken concurrently (proposed
paragraph 47B(2)(b) of the RIHE Act). The reviewers will be required
to provide a report of the review to the Minister for presentation to
Parliament (proposed subsection 25(3) of the PHCR Act and proposed
subsection 47B(3) of the RIHE Act). The report must be completed
within 12 months after the end of the relevant seven-year period (proposed subsection
25(4) of the PHCR Act and proposed subsection 47B(4) of the RIHE
Act). The people undertaking the review must consult and set out the views
in the report for the Commonwealth and the states and territories as well as a
broad range of people with relevant expertise or experience (proposed
subsection 25(5) of the PHCR Act and proposed subsection 47B(5)
of the RIHE Act).
One report may be provided for the reviews under both Acts
(proposed subsection 47B(6) of the RIHE Act).
Other provisions
Changes to the Freedom of Information Act 1982
Items 21, 22 and 23 make changes to the Freedom of
Information Act 1982 (FOI Act) to ensure that information
recorded on the register remains private and cannot be accessed under the FOI Act.
Application
and transitional provisions
NHMRC
Licensing Committee reports to Parliament
Under current subsection 19(3) of the RIHE Act, the NHMRC Licensing Committee must
table reports in Parliament. These reports include information about the
operation of that RIHE Act, and the licences issued under it.
Item 117 will change the operation of subsection
19(3) for a transitional period so that information about the operation of new
provisions of the RIHE Act, and information about mitochondrial donation
licences, will not have to be provided in the NHMRC Licensing Committee’s
reports for a period of six months after the commencement of Schedule 1. However,
this information must be included in the next report.
Given that the NHMRC Licensing Committee must table
reports in Parliament on fixed dates, and it cannot be known on what date the
amendments made by Schedule 1 will commence, there may not be sufficient time
to prepare the report as required by section 19. This transitional amendment
has been made to provide ample time to prepare a report including the new
amendments.
Pre-commencement
licence applications
Item 118 is a transitional provision that applies
to applications for licences, made under subsection 20(1) of the RIHE
Act, that have not been processed or finalised before the commencement of
Schedule 1 to the Bill. For such licence applications, the following documents
that would have been applicable but for the Bill continue to be relevant:
- current
paragraph 21(3)(c) of the RIHE Act
- the
following provisions of the Regulations:
- the
current definitions of the ART Guidelines and the National Statement in section
5
- current
section 7 ‘Definition of proper consent—prescribed guidelines’, and
- current
section 9 ‘Determination by NHMRC Licensing Committee of licence
applications—prescribed guidelines’.
Concluding
comments
The Mitochondrial Donation Law Reform (Maeve’s Law) Bill
2021 will allow, for the first time, embryos to be created for reproductive
purposes that contain the genetic material from more than two people and have
heritable changes to their genome. If the Bill is passed, women who are at high
risk of having children with serious mitochondrial disease will be able to consider
mitochondrial donation as an option for having children. Initially this would be
by participating in a clinical trial and it may later be available in a
clinical practice setting.
Mitochondrial donation will be introduced in two stages,
with Stage 1 anticipated to take 10 years, with a review scheduled to take
place seven years after the commencement of the Act. Stage 1 will allow the
Embryo Research Licensing Committee of the NHMRC to authorise three of the five
types of mitochondrial donation licences, including a clinical trial licence. If
Stage 2 is implemented as anticipated, the remaining two licences would be
available and would allow for use of the approved technique in a clinical
setting, making mitochondrial donation more widely available. Stage 2 will
require further legislative changes, including to state or territory legislation
where the clinical practice activities would take place.
There are complex and complicated issues surrounding the
introduction of mitochondrial donation, as, not only is it scientifically a
relatively new technology with new evidence emerging and known knowledge gaps,
it also raises a number of ethical and social considerations. There are people who
strongly support the introduction of mitochondrial donation and there are
people who strongly oppose its introduction. Considerations include the use and
status of human embryos, the health and wellbeing of the child, the rights and
impact on the women who donate their eggs, and broader community considerations,
such as equitable access. Given the complexities of the issues, the Government
will call for a conscience vote on the Bill.
Appendix
Table 6: definitions
added to the RIHE Act and the RIHE Regulations
| Term |
Definition |
Bill item number |
|
Clinical practice licence
|
A licence referred to in proposed section 28G.
|
Item 10
|
|
Clinical practice research and training licence
|
A licence referred to in proposed section 28F.
|
Item 10
|
|
Clinical trial licence
|
A licence referred to in proposed section 28E.
|
Item 10
|
|
Clinical trial research and training licence
|
A licence referred to in proposed section 28D.
|
Item 10
|
|
Constitutional corporation
|
Constitutional corporation means a trading, foreign or
financial corporation within the meaning of paragraph 51(xx) of the Constitution.
|
Item 9
|
|
Donor
|
If a particular use of a mitochondrial donation technique
results in the creation of a zygote that:
- has
nuclear DNA from a woman and a man and
- contains
mitochondria from a different woman.
The woman mentioned in paragraph (b) is the donor in
relation to that use of the technique.
|
Items 10 and 17 (proposed subsection 28R(2))
|
|
General licence
|
A licence issued under section 21.
|
Item 10
|
|
Mitochondrial donation licence
|
- a
pre-clinical research and training licence
- a
clinical trial research and training licence
- a
clinical trial licence
- a
clinical practice research and training licence
- a
clinical practice licence.
|
Item 10
|
|
Mitochondrial donation technique
|
A technique, prescribed by the Regulations for the
purposes of this definition, that:
- can
be used to minimise the risk of a woman's offspring inheriting mitochondrial from
that woman that would predispose the offspring to mitochondrial disease and
- involves
the use of ART to create a zygote that has the parental nDNA and donor
mitochondria and
- does
not involve:
- intentionally modifying nDNA or mtDNA or
-
using any animal cell or cell component or
-
creating a chimeric embryo or hybrid embryo.[193]
|
Item 10
|
|
National Statement
|
The National
Statement on Ethical Conduct in Human Research, issued by the CEO of
the NHMRC under the National Health
and Medical Research Council Act 1992.
|
Item 40
|
|
Patient
|
A woman whose pregnancy is sought to be achieved using a
mitochondrial donation technique under a clinical practice licence.[194]
|
Item 10
|
|
Permitted technique (for a mitochondrial donation licence)
|
A mitochondrial donation technique that declared by the
Regulations as permitted to be used under a specific mitochondrial donation
licence (outlined in the proposed changes to the Regulations).
|
Item 10
|
|
Pre-clinical research and training licence
|
A licence referred to in proposed section 28C.
|
Item 10
|
|
Proper consent for the purposes of Division
4 (general licences) of Part 2 of the RIHE Act
|
Consent that is obtained in accordance with the Ethical guidelines on the use of
assisted reproductive technology (ART Guidelines) issued by the CEO
of the NHMRC under the National Health and Medical Research Council Act
1992.
|
Item 41 (proposed definition of proper consent at
section 8 of the RIHE Act); item 71 (proposed subsection 24(9) of the RIHE
Act) and item 112 (proposed amendment of section 7 of the RIHE
Regulations).
|
|
Proper consent for the purposes of
proposed Division 4A (mitochondrial donation licences) of Part 2 of the RIHE
Act
|
Consent:
|
Item 17 (proposed subsection 28N(8) of the RIHE Act;
item 20 (proposed subsection 7J of the RIHE Regulations); and item 41
(proposed definition of proper consent at section 8 of the RIHE Act).
|
|
Responsible person for the purposes of
Division 4 (general licences) of Part 2
|
- in
relation to an excess ART embryo:
-
each person who provided the egg or sperm from which the embryo was
created; and
-
the woman for whom the embryo was created, for the purpose of achieving
her pregnancy; and
-
any person who was the spouse of a person mentioned in subparagraph
(i) at the time the egg or sperm mentioned in that subparagraph was provided;
and
-
any person who was the spouse of the woman mentioned in subparagraph
(ii) at the time the embryo was created; or
- in
relation to an embryo other than an excess ART embryo - each person whose
reproductive material, genetic material or cell was used, or is proposed to
be used, in the creation or use of the embryo; or
- in
relation to a human egg—the woman who was the biological donor of the egg.
|
Item 42 (proposed definition of responsible person
at section 8 of the RIHE Act); item 71 (proposed subsection 24(9) of
the RIHE Act)
|
|
Responsible person for the purposes of Division
4A of Part 2
|
Responsible person in relation to a human egg or a
human sperm is the person who is the biological donor of the human egg or
human sperm.
|
Item 17 (proposed subsection 28N(8)); item 42 (proposed
definition of responsible person at section 8 of the RIHE Act).
|
|
Trial participant
|
A woman whose pregnancy is sought to be achieved using a
mitochondrial donation technique under a clinical trial licence.
|
Item 10
|
Source: Mitochondrial Donation Law Reform
(Maeve’s Law) Bill 2021.