This Bills Digest replaces a preliminary Digest published on 25 March 2024 to assist in early consideration of the Bill.
Key points
- The Therapeutic Goods and Other Legislation Amendment (Vaping Reforms) Bill 2024 amends the Therapeutic Goods Act 1989 and other Commonwealth Acts to prohibit the importation, domestic manufacture, supply, commercial possession and advertisement of non-therapeutic and disposable vaping goods.
- The Bill does not prohibit vaping but rather vapes will be regulated as therapeutic goods and will only be supplied by registered pharmacists, medical practitioners or nurse practitioners authorised to do so under state or territory law.
- The Bill will also expand the definition of ‘therapeutic goods’ and provide for a range of new enforcement powers, including a new power for the Secretary to issue enforceable directions and a broad power in relation to the seizure and destruction of goods.
- Vaping rates are increasing in Australia, particularly among youth and young adults, including those who have never smoked. Vaping is associated with a number of adverse health effects and the long-term health impact remains unknown.
- The vaping reform measures are intended to reduce the prevalence of smoking and vaping in the community, in line with the objectives, priorities and targets outlined in the National Tobacco Strategy 2023–2030. The Bill seeks to implement the second stage of the Government’s vaping reform measures.
- The Senate Community Affairs Legislation Committee conducted an inquiry into the Bill and recommended that the Bill be passed. Submissions from health organisations and public health researchers strongly support the Bill, while submissions from retail organisations and individual retailers broadly support a regulatory, rather than prescription only model.
- The Nationals have stated they do not support the Bill. Members of the Liberal Party of Australia and the Australian Greens on the Senate Community Affairs Legislation Committee recommended passing the Bill but noted a number of concerns and are yet to make a formal announcement on their position.
Introductory Info
Date introduced: 21 March 2024
House: House of Representatives
Portfolio: Health and Aged Care
Commencement: Schedules 1 and 3 commence on the later of 1 July 2024 or the day after Royal Assent; Schedule 2 commences the day after Royal Assent.
Purpose of
the Bill
The purpose of the Therapeutic
Goods and Other Legislation Amendment (Vaping Reforms) Bill 2024 (the Bill)
is to amend the Therapeutic
Goods Act 1989 and other Commonwealth Acts to prohibit the importation,
domestic manufacture, supply, commercial possession and advertisement of
non-therapeutic and disposable vaping goods.
The Bill proposes
to create ‘a single consistent framework that applies nationally to regulate
the importation, domestic manufacture, supply, commercial possession and
advertisement of all vapes, irrespective of nicotine content or therapeutic
claims’.[1]
Under this framework, vapes will be regulated as therapeutic goods and will
only be supplied by registered pharmacists, medical practitioners or nurse
practitioners authorised to do so under state or territory law.
The Bill also introduces criminal and civil penalty
provisions relating to the importation, domestic manufacture, supply,
commercial possession and advertisement of vaping goods. This includes an
exception for personal use relating to the possession of less than a
‘commercial quantity’ of vaping goods (with ‘commercial quantity’ to be
prescribed in the regulations).
The measures introduced in the Bill are intended to reduce
the prevalence of smoking and vaping in the community, as well as prevent the
uptake of vaping among people who have never smoked, in line with the
objectives, priorities and targets outlined in the National
Tobacco Strategy 2023–2030. The Bill does not prohibit vaping. Access
to therapeutic vapes for smoking cessation purposes under medical supervision
will be maintained.
Background
What are vapes?
The Department
of Health and Aged Care (DHAC) defines electronic cigarettes (e-cigarettes)
as battery‑operated devices that deliver an aerosol by heating a solution
that users of the e-cigarette then breathe in.[2]
As the aerosol is commonly referred to as ‘vapour’, using e-cigarettes is often
referred to as ‘vaping’ and e-cigarettes are more commonly referred to as
vapes.
Vapes come in two main configurations:
- a
disposable single use (closed system) vape that is pre-filled with an e-liquid,
cannot be refilled and is disposed of once the vaping substance runs out
- a
refillable/reusable vape that may be refilled or reused multiple times, with
various e-liquids pods, cartridges or solutions sold separately to the device;
these vapes are intended to be recharged and reused.[3]
DHAC notes that the liquids contained in vapes may contain
a range of chemicals, including nicotine.[4]
The ways in which e-cigarettes are currently regulated in Australia depends on
whether they contain nicotine; however, due to incorrect and incomplete
labelling, the ingredients of e‑cigarettes are not always easily
identifiable which can make regulation difficult.
Use of vapes
Vapes first began to be used in Australia in the mid-2000s
and in recent years the number of people using vapes has rapidly increased. The
National
Drug Strategy Household Survey (NDSHS) and the Australian
Secondary Students’ Alcohol and Drug Survey (ASSAD) are key sources of data
on the use of vapes and vaping products in Australia. The most recent NDSHS and
ASSAD data are for 2022–23.
According to the NDSHS, in
2022–23, 7% (1.5 million people) of Australians aged 14 and older were
currently using vapes. This represents a three-fold increase on the figure for
2019 (2.5%).
While vaping increased in all age groups, the increase was
particularly pronounced among younger Australians. Current use of vapes
quadrupled between 2019 and 2022–23 among people aged 18–24 (from 5.3% to 21%)
and increased more than five-fold—albeit from a lower base—among people aged
14–17 (from 1.8% to 9.7%). The most common reason people gave for first using a
vape was ‘out of curiosity’, while one-in-five people who had ever used vapes
reported doing so to help them quit smoking.
Despite it now being illegal to obtain vapes containing
nicotine without a prescription, in 2022–23 around three-quarters of people
currently using vapes reported that the last one they used contained nicotine.
Most people who had used vapes with nicotine (87%) reported that they had
obtained them without a prescription.
In keeping with latest NDSHS findings, ASSAD data for
2022–23 show that the number of 14–17 year-olds using vapes has increased
significantly in recent years.
In 2022–23 just under one-third (30%) of Australian
secondary school students had ever vaped. This represents an increase on
equivalent figures for 2014 and 2017 (13% and 14% respectively). Similarly,
past month vaping was significantly higher in 2022–23 (16%) when compared to
2014 and 2017 (3% and 4% respectively).[5]
Of students who had ever vaped, more than two‑thirds (69%) reported never
having smoked a tobacco cigarette before their first vape.[6]
While smoking has generally decreased among Australian
secondary school students over time, ASSAD researchers expressed concerns that
there was an increase in susceptibility to smoking among Australian secondary
school students who have never smoked between 2017 and 2022–23.[7]
One-in-five students (20%) who had never smoked prior to trying an e-cigarette
reported subsequent smoking (that is, at least a few puffs) of tobacco
cigarettes.[8]
In addition to capturing data on the use of vapes, the
NDSHS surveyed
participants on their support for a range of policy measures to address
vaping. There was significant support (over 60%) in all age groups for:
- banning
additives in e‑cigarettes, to make them less attractive to young people (78%)
- restricting
the use of e‑cigarettes in public places: 80% (an increase from 69% in
2019)
- prohibiting
the sale of e‑cigarettes, including those without nicotine, to people
under 18 years of age: 86% (an increase from 79% in 2019)
- strengthening
restrictions on the advertising and promotion of e‑cigarettes: 82% (an
increase from 67% in 2019).
Support for these policy measures was highest among people
aged 60 and over, and lowest among people aged 14–29.
Health impacts of vaping
While vaping is generally reported to be less harmful than
smoking, it should not be considered safe, particularly for non-smokers and
young people. Vaping is associated with a range of adverse health outcomes,
such as poisoning, nicotine toxicity, burns, throat and mouth irritation,
cough, headache, nausea, and dizziness. Vapes that contain nicotine can cause
dependence or addiction in non-smokers and may lead to smoking uptake.[9]
A recently published systematic
review of health outcomes associated with e-cigarettes, funded by the
Department of Health and Aged Care and the National Health and Medical Council,
found the following:
Evidence regarding
the health effects of e-cigarettes is very limited. The risks of several
adverse health outcomes are higher in e-cigarette users. There is conclusive
evidence that nicotine e-cigarettes can cause poisoning and immediate
inhalation toxicity (including seizures), particularly in children and
adolescents, and that malfunctioning devices can cause injuries and burns;
there is substantial evidence that nicotine e-cigarettes can cause dependence
or addiction in non-smokers
…
There is moderate
evidence that nicotine e-cigarettes can cause less serious adverse events, such
as headache, cough, throat irritation, dizziness, and nausea.
The study
concluded:
E-cigarettes can
be harmful to health, particularly for non-smokers and children, adolescents,
and young adults. Their effects on many important health outcomes are
uncertain. E-cigarettes may be beneficial for smokers who use them to
completely and promptly quit smoking, but they are not currently approved
smoking cessation aids. Better quality evidence is needed regarding the health
impact of e‑cigarette use, their safety and efficacy for smoking
cessation, and effective regulation.[10]
Tobacco
in Australia also provides a summary of the evidence on the relationship between e‑cigarette
use and a range of health issues such as pregnancy outcomes, brain development,
cardiovascular disease risk, cancer risk and oral health. The authors state the
following regarding vaping and health:
Due to their
relatively recent introduction onto the market, most of the known health
effects of e‑cigarettes have arisen from short term use. As e-cigarettes
contain some of the same toxic chemicals present in cigarette smoke, there is
concern that health effects will manifest in the future as a result of
long-term use, in a similar manner to those caused by tobacco products. These
may include cancer, cardiovascular disease, chronic obstructive pulmonary
disease and periodontitis, as discussed in this section.[11]
Research on vaping and health continues to be published on
a regular basis. Currently the long‑term health impact of vaping remains
unknown.
Regulation of vapes prior to the recent reforms
Nicotine vapes
In Australia, responsibility for the regulation of
medicines, poisons and therapeutic goods is shared between state, territory and
Commonwealth governments. Currently Commonwealth law prevents the importation,
and state and territory laws prevent the domestic supply, of nicotine vapes
without a prescription.
Medicines
are classified into Schedules which determine how freely they will be
available to the public according to risk (for example, where you can purchase
them from and whether you need a prescription).[12]
The Schedules are published in the Poisons Standard made
under the Therapeutic Goods Act and are given legal effect through state
and territory legislation.
The Secretary of the DHAC makes decisions on the
scheduling of medicines (and chemicals), and other changes to the Poisons
Standard. On 21 December 2020, a delegate of the Secretary of DHAC made
a final decision to list the regulation of nicotine as a Schedule 4
medicine except where it occurs:
- in
tobacco prepared and packed for smoking or
- in
preparations for oromucosal or transdermal administration (such as nicotine
patches, gum, mouth spray, lozenges and inhalators) that are used as an aid in
withdrawal from tobacco smoking.[13]
This decision took effect on 1 October 2021. The
scheduling of nicotine vaping products as a Schedule 4 medicine meant that a
prescription from a medical practitioner was required to access nicotine vaping
products (including when they were being imported) and they could only be
lawfully dispensed in Australia by pharmacists under state/territory legislation.
These reforms also make it unlawful to import vapes containing nicotine without
a valid prescription from a medical practitioner.[14]
Outside of these products, the Poisons Standard lists
nicotine for human use as a Schedule 7 poison. Schedule 7 poisons are
substances not for general sale by retail and under state/territory legislation
it is an offence to manufacture, sell or supply nicotine as a Schedule 7 poison
without a licence or specific authorisation. Schedule 7 nicotine does not
include tobacco when prepared and packed for smoking or e-cigarette products
that are included in Schedule 4 and available on a prescription.
State and territory laws vary when it comes to
unauthorised possession of prescription medicines. In most
jurisdictions, the possession of a prescription only substance without a
valid prescription amounts to an offence:
It follows that in most jurisdictions, the possession of
nicotine vaping products without a prescription is technically an offence
(under the relevant state or territory drugs and poisons legislation). These
offences are not new, and the penalties they impose relate to the
possession/use of any prescription-only substance (and not specifically
to nicotine vaping products).[15]
For example, in New South Wales, section 16 of the Poisons
and Therapeutic Goods Act 1966 (NSW) provides that it is an offence to
possess a restricted substance unless authorised
or where it is has been lawfully prescribed (which includes nicotine as a
Schedule 4 product), with a maximum penalty of a fine of 20 penalty units[16]
($2,200) or 6 months imprisonment or both (paragraph 16(1)(b)). Section 9 of
the Poisons and Therapeutic Goods Act 1966 (NSW) provides that is
an offence to supply a restricted substance for therapeutic use except in
accordance with the relevant wholesaler’s license or authority with a maximum
penalty of a fine of 15 penalty units ($1,650) or 6 months imprisonment or both
(paragraph 9(1)(b)). NSW Health reports on the number
of prosecutions for the illegal sale of nicotine containing e-cigarettes
and other nicotine products, with there being 6 successful prosecutions in
2023.
Normally, prescription medicines can only be
supplied in Australia if they have been approved by the Therapeutic Goods
Administration (TGA), following an assessment of their safety, quality and
efficacy, and they have been included in the Australian Register of Therapeutic
Goods (ARTG), or a lawful exception to registration applies. Criminal
offences and civil penalties are set out in the Therapeutic Goods Act for importing,
exporting, supplying and manufacturing therapeutic goods and medical devices
not included in the Register (these are summarised at pages 43–44 of the Explanatory
Memorandum to the Bill).
As there are currently
no nicotine vapes registered on the ARTG, nicotine vapes have been supplied
to patients with a prescription as ‘unapproved therapeutic goods’ via the
following pathways:
- Personal
Importation Scheme: This scheme allows a person with a prescription from an
Australian medical practitioner to directly import up to 3 months’ supply of
nicotine vaping products (NVPs) per order for their own personal use. A person
can also import NVPs for immediate family members who have a prescription.
- Authorised
Prescriber Scheme (AP scheme): Under this scheme, a medical practitioner
can apply to the TGA for approval to supply NVPs to their patients as an aid to
stop smoking.
- Special
Access Scheme B (SAS B): Under this scheme, a medical practitioner can
apply to the TGA to supply NVPs to a single patient on a case-by-case basis.[17]
The TGA also introduced the TGO 110 Standard
for Nicotine Vaping Products (as made) supplied domestically within
Australia to align the 2021 scheduling change which sets out the minimum safety
and product requirements with respect to NVPs.
Vapes that do not contain
nicotine
Prior to the recent reforms, vapes which did not contain
nicotine were not regulated under the Therapeutic Goods Act, unless
represented to be for therapeutic use. As stated by the TGA, vaping devices
were also excluded from the operation of the Act ‘unless they are intended
exclusively for the vaporisation and administration by inhalation of a
medicine’.[18]
There was therefore no product standard that applied to these vapes.
Currently
all states and territories restrict the supply of vapes that do not contain
nicotine, through tobacco control laws and/or public health laws, though
regulation ‘is limited and inconsistent’:
All ban the supply of vapes to minors and the use of vapes in
smokefree areas. All regulate the advertising, display and marketing of vapes.
Most states and territories (except Victoria and Queensland) require either a
licence or a retailer identification number for retail sale. The controls in
Western Australia are more extensive than other jurisdictions. The Tobacco
Products Control Act [2006] (WA) makes it an offence to sell any food, toy
or other product that is designed to resemble a tobacco product or package or
is in packaging that is designed to resemble a tobacco product or package,
regardless of whether it contains nicotine. Western Australia also permits
registered pharmacists to supply nicotine vapes as part of a medically
supervised smoking cessation program.[19]
Vaping reforms
In November 2022, the Minister for Health and Aged Care
Mark Butler announced a public
consultation process on the regulation of nicotine vaping products to
be run from 30 November 2022 to 16 January 2023 by the TGA.[20]
The public consultation process was instigated largely in response to evidence
of an increase in e-cigarette use by children.
Following the TGA consultation process, Mr Butler announced in
May 2023 that the Government, subject to consultation with states and
territories, would be proposing stronger regulation and enforcement of all vaping
products, ‘including new controls on their importation, contents and
packaging’.
On 1 September 2023, Commonwealth
and state/territory Health Ministers ‘agreed to extend the operation of the
Therapeutic Goods Act to restrict the importation, manufacture, and
supply of all vapes’.[21]
Further details about these reforms were announced
on 28 November 2023.[22]
In December 2023, the Government made amendments to three
regulations and nine new or revised legislative instruments under the Therapeutic
Goods Act and the Customs
(Prohibited Imports) Regulations 1956 to give effect to the first
stage of these reforms:
From 1 January 2024:
the importation of all disposable vapes is banned, with very
limited exceptions
- the Special Access Scheme C (SAS
C) pathway, is available to facilitate legitimate patient access to therapeutic
vapes, for smoking cessation and the management of nicotine dependence
- a form for importers and
manufacturers of therapeutic vapes is available to notify the TGA about
compliance with the relevant product standards prior to importation into
Australia, or release for supply of vapes manufactured domestically (notices
are required for goods imported or released for supply on or after 1 March
2024)
- an application form for
therapeutic vape importers is available to apply for licences and permits for
importing therapeutic vapes (licences and permits are required for goods
imported on or after 1 March 2024).
From 1 March 2024:
- the importation of all vapes is
banned unless importers have an import licence and permit from the Office of Drug Control
- therapeutic vape importers and
manufacturers are required to notify the TGA about compliance with the relevant
product standards before importation to Australia or release for supply in
Australia
- the Personal Importation Scheme
for vapes is closed
- travellers may bring a small
quantity of vapes into Australia
- some changes to the quality
requirements for therapeutic vapes for smoking cessation and the management of
nicotine dependence, including restrictions on flavours to mint, menthol and
tobacco
- a new medical device standard
applies to therapeutic vaping devices that were previously excluded from the
therapeutic goods framework.
Further information about these reforms is set out in
detail in the Impact
Analysis prepared by the TGA published in October 2023.
Prescribing pathways since 1 January 2024
As noted above, nicotine vapes have previously been supplied
to patients with a prescription as ‘unapproved therapeutic goods’ via the Personal
Importation Scheme (which closed on 1 March 2024 for vaping products), the Authorised
Prescriber Scheme and the Special
Access Scheme B (SAS B).
Since 1 January 2024, prescribers have also been able to
prescribe therapeutic vapes to patients for smoking cessation or the management
of nicotine dependence via Special
Access Scheme C (SAS C). Products that can be supplied via the SAS C
pathway are those that are deemed to have an ‘established history of use’.
Under SAS C, prescribers can access an unapproved product immediately, without
applying to the TGA for pre-authority or approval. Notification that a product
has been prescribed under SAS C needs to be provided to the TGA within 28 days
of use.
If the Bill is passed, prescribers will still be able to
use the Authorised Prescriber Scheme, SAS B and SAS C to prescribe vaping
products to patients (see Figure 1). In an overview
of the regulatory changes under the proposed reforms presented by the TGA, it
was noted that the SAS C pathway is intended to help reduce the administrative
burden on doctors and nurse practitioners prescribing therapeutic vapes:
Doctors and nurse practitioners can prescribe therapeutic
vapes for smoking cessation or the management of nicotine dependence through
Special Access Scheme C (SAS C).
To reduce the administrative burden on prescribers:
- Doctors and nurse practitioners
will be able to prescribe vapes through the SAS C pathway without applying to
the TGA for pre-authority or approval.
- The SAS C notification process
has been streamlined to help prescribers manage increased demand for
prescriptions.
Doctors may also prescribe through the Authorise Prescriber
(AP) Scheme
The SAS B may also be used.[23]
When prescribing and dispensing therapeutic vapes,
prescribers and pharmacists must do so in accordance with relevant state of
territory legislation.
To assist prescribers in determining when to prescribe
therapeutic vapes, the Royal Australian College of General Practitioners
(RACGP) are currently updating their guidelines
for supporting smoking cessation (first published in 2011), to include new
guidance on prescribing vapes. Provisional
guidance, Supporting smoking cessation: A guide for health
professionals, Guidance updates on smoking and vaping cessation support related
to Australia’s vaping regulation, was published in December
2023.
Figure 1 – Pathways to prescribe therapeutic vapes
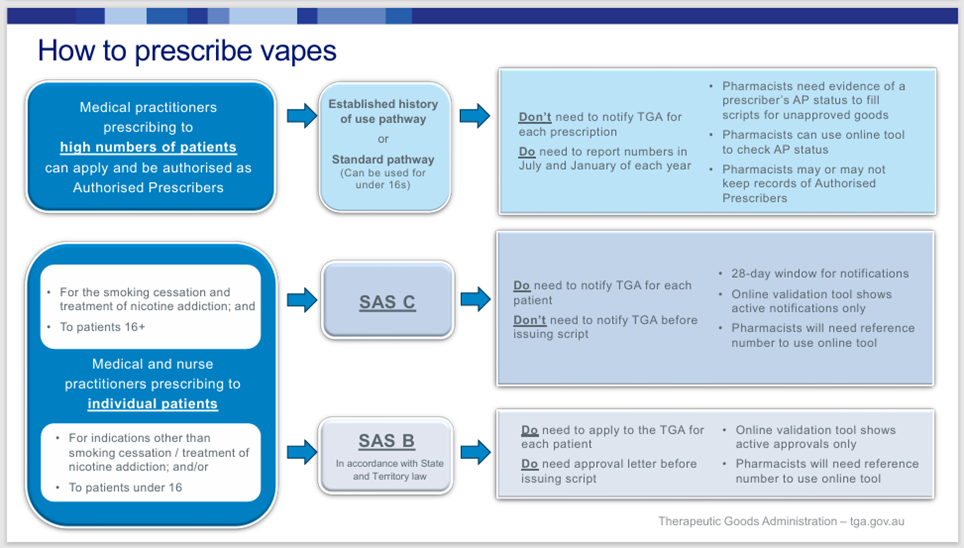
Source: TGA, Royal Australian College of General
Practitioners and Quit Centre, Prescribing Therapeutic Vapes: Overview of Regulatory
Changes, Webinar, accessed 6 May
2024, slide 13.
International
regulation of vapes
Regulation of e-cigarettes and vaping products varies
internationally. Tobacco
in Australia has published an overview of regulation in other
jurisdictions (updated September 2022), which includes information on the sale
of vapes to minors, the regulation of e-liquid ingredients (including
nicotine), restrictions on advertising and promotion, and taxes on vapes.[24]
The Global State of
Tobacco Harm Reduction (GSTHR) provides a country-by country summary on the
use, availability and regulations applying to vaping products, oral tobacco and
heated tobacco products.
According to the UK
House of Commons Library ‘some countries, including Brazil and Singapore,
have adopted neither a medicine nor consumer product approach to regulation,
instead issuing an outright ban on the sale, distribution and importation of
e-cigarettes’.[25]
The World
Health Organization reported in 2022 that ‘121 countries have adopted
measures addressing ENDS [electronic nicotine delivery systems]’, with 34
countries currently banning the sale of ENDS and 87 countries allowing the sale
of ENDS though have adopted one or more measures either fully or partially to
regulate them:
ENDS are regulated in: Albania, Algeria, Andorra,
Armenia, Australia, Austria, Azerbaijan, Bahrain, Barbados, Belarus, Belgium,
Benin, Bolivia (Plurinational State of), Bulgaria, Cameroon, Canada, Chile,
China, Congo, Costa Rica, Côte d’Ivoire, Croatia, Cyprus, Czechia, Denmark,
Dominican Republic, Ecuador, Egypt, El Salvador, Estonia, Fiji, Finland,
France, Georgia, Germany, Greece, Guyana, Honduras, Hungary, Iceland, Ireland,
Israel, Italy, Jamaica, Kazakhstan, Kenya, Kuwait, Kyrgyzstan, Latvia, Lebanon,
Lithuania, Luxembourg, Malta, Monaco, Montenegro, Nepal, Netherlands (Kingdom
of the), New Zealand, Niue, North Macedonia, Palau, Papua New Guinea, Paraguay,
Philippines, Poland, Portugal, Republic of Korea, Republic of Moldova, Romania,
Russian Federation, Saint Lucia, Samoa, San Marino, Saudi Arabia, Serbia,
Slovakia, Slovenia, Spain, Sweden, Tajikistan, Togo, Tuvalu, Ukraine, United
Arab Emirates, United Kingdom, the United States, Uzbekistan
ENDS is banned in: Argentina, Brazil, Brunei
Darussalam, Cabo Verde, Cambodia, Democratic People’s Republic of Korea,
Ethiopia, Gambia, India, Iran (Islamic Republic of), Iraq, Jordan, Lao People’s
Democratic Republic, Malaysia, Mauritius, Mexico, Nicaragua, Norway, occupied
Palestinian territory, Oman, Panama, Qatar, Singapore, Sri Lanka, Suriname,
Syrian Arab Republic, Thailand, Timor-Leste, Türkiye, Turkmenistan, Uganda,
Uruguay, Vanuatu, and Venezuela (Bolivarian Republic of).[26]
Canada
In Canada, the ownership and possession of vapes is not
prohibited and they are available for sale as a consumer product to adults. The
relevant federal legislation, the Tobacco
and Vaping Products Act (S.C. 1997, c. 13) and related regulations,
provides a broad regulatory framework for the manufacture, sale, labelling and
promotion of vaping products. As such, regulation of vapes is predominantly the
responsibility of the Provinces and Territories which have each enacted their
own Acts and Regulations. These prescribe minimum legal ages for sale and may
also contain provisions regarding the display, advertisement, promotion, place
of sale, premises where vapes may be used and prohibitions on flavours of
vapes.
New Zealand
In New Zealand, vapes are available as a consumer product
to adults. The sale, advertising, and use of e-cigarettes and nicotine liquids
(vaping products) is largely regulated under the Smokefree
Environments and Regulated Products Act 1990 (NZ) and associated
regulations. The regulation of vaping products falls to the Ministry of
Health’s Vaping
Regulatory Authority and all vapes must be notified to the Authority before
they can be sold. New requirements for vaping products, including restrictions
on flavours, requirements for specialist vape retailers and nicotine limits, came
into effect on 21 March 2024 and the New Zealand Government recently
announced it would ban disposable vapes.
United Kingdom
The Tobacco Products Directive 2014/14/EU (TPD) was
passed by the European Parliament and became law in April 2014. The
requirements for e-cigarettes set out in Article 20 of the TPD cover product
standards and nicotine strength; safety; labelling and packaging, notification
and vigilance; advertising and annual reporting. Member States had until May
2016 to transpose the new rules into national law (including establishing
relevant offences under their national law).
In the UK, vapes are regulated as both consumer and
therapeutic products, but all supply is currently through the consumer market.
The Tobacco and Related Products Regulations 2016 (which
implemented the TPD into law) impose product standards for nicotine vapes.
These include restrictions on maximum nicotine strength, refill bottle and tank
size limits, and prohibit in certain media the advertising of
nicotine-containing e-cigarettes and e-liquids sold as consumer goods. The
Regulations do not apply to vapes that do not contain nicotine. These are
covered by the General
Products Safety Regulations 2005 which sets out general product safety
standards. Currently under 18s may purchase non-nicotine vapes, although the UK
Government is considering
changing this and has recently announced
a ban on disposable vapes, along with other measures to prevent youth vaping.[27]
Committee consideration
On 7 March 2024, the Senate
referred the provisions of the Bill to the Senate Community Affairs Legislation
Committee for inquiry and report by 8 May 2024. Public hearings for the inquiry
were held on 1 May 2024 and 2 May 2024. Further information about the inquiry
is available on the Committee’s
homepage.
As of 3 May 2024, over 200 submissions had been received and
published by the Committee. Submissions from health organisations and public
health researchers are strongly supportive of the Bill, while submissions from
retail organisations and individual retailers are broadly in support of a
regulatory, rather than prescription only model. A number of submissions
received from individuals who are current vape users also supported a
regulatory model, with some expressing concerns about continued access to vaping
products if the Bill is passed. Further information can be found in the
‘Position of major interest groups’ section below.
On 9 May 2024, the Committee tabled its report
recommending that the Bill be passed.[28]
Coalition Senators who participated in the inquiry and the Australian Greens
both provided additional comments on the Bill, while National Party Senators
provided a dissenting report (see the policy position of non-government
parties/independents below).
Senate Standing Committee for the Scrutiny of Bills
The Senate Standing Committee for the Scrutiny of Bills
raised a number of issues with respect to the Bill, including:
- the
inclusion of significant matters in delegated legislation
- the
imposition of strict liability on offences with higher than 60 penalty units
- the
use of offence-specific excuses which reverse the evidential burden of proof
- the
provision of a broad discretionary power to allow the Secretary of DHAC to
consent to the manufacture, supply or possession of vaping goods, or to refuse
such an application, or grant it subject to conditions
- whether
independent merits review is available for directions issued under proposed
subsection 42YT(2) of the Bill
- the
new power to seize assets and whether use and derivative use immunity applies
in relation to incidentally seized materials
- the
appropriate delegation of administrative powers and functions.[29]
Some of these issues are discussed further in the ‘Key
provisions and issues’ section of this Bills Digest.
The Minister provided a response
to the Committee on 9 April 2024, however at the time of writing it has not
been published.
Policy position of
non-government parties/independents
Liberal Party of Australia
The Liberal Party is yet to formally announce its position
on the Bill.
When the Bill was introduced, the Deputy Leader of the
Opposition, Sussan Ley stated
that the Coalition supported scrutiny of the Bill by a Senate Committee and
expressed concerns about regulation of the illicit tobacco and e-cigarette
market if the Bill were to be passed.[30]
Prior to the Bill’s introduction, the Shadow Minister for Health and Aged Care, Anne Ruston commented that ‘the Coalition is still working through the details
of the legislation’ and expressed concerns that the current model ‘will
not prevent children from having access to vaping products and will further
drive the sale of these products to the black market’.[31]
She has previously indicated
that the Opposition is open to ‘all sensible and workable options that will prevent
children from getting access and becoming addicted to vaping’.[32] More
recent media reports suggest
that the Liberal Party is leaning towards supporting a regulatory model, in
which vaping products are taxed and regulated in a similar manner to tobacco
products. In a radio interview the Opposition Leader Peter Dutton, stated
that his instinct is to ‘treat it [vapes] the same as tobacco’.[33]
In their additional comments to the Senate Committee’s
report into the Bill, Coalition Senators stated that they ‘note the
recommendations of the majority report and reserve our final position while
this policy makes its way through our internal processes’.[34]
While supportive of the policy intent of the Bill to combat youth vaping,
Coalition Senators argued that the current, and proposed prescription model in
the Bill, ‘has failed to address the exponential growth in underage vaping’ and
raised concerns that the Bill would contribute to black market activity
regarding the sale of both illicit tobacco and illicit e-cigarettes.[35]
The Nationals
The Nationals do not support the Bill, instead favouring a
regulatory approach. In October 2023, the leader of the National Party, David
Littleproud, called on the Government to
‘urgently regulate and tax e-cigarettes’ to address increasing vaping rates. In
the same media release, the Leader of the Nationals stated:
‘... [t]he prescription-only model is not working and it’s
time to stamp out the illegal black market and move in a constructive way
towards regulation to protect children.
…
Licencing retailers for e-cigarettes, selling only to age 18
and above, removing flavours that are deemed attractive to children, while also
ensuring harsh penalties for those illegally selling products, should be
trialled, because prohibition isn’t working’.[36]
The preference for a regulatory approach was reiterated
when the Bill was introduced, with the member for Cowper, Pat Conaghan stating:
We [The Nationals]
believe that vaping needs to be viewed in the full context of law enforcement
and harm reduction, by reducing the impacts and cost burden of traditional
cigarettes and smoking on our health system, stopping the funding of organised
crime organisations, stopping the availability of unregulated and unsafe
products to the community, implementing plain packaging, regulating flavours,
enforcing market bans and issuing stronger enforceable penalties for those
caught conducting businesses outside those parameters.[37]
This preference was also reflected in the National Party
Senators’ dissenting report on the Bill, which recommended that the Bill not be
passed and instead argued that vaping products should be regulated in the same
way as tobacco.[38]
The Nationals have previously
stated that under their proposal any revenue
generated through taxes on vapes would be directed towards regional healthcare
and this suggestion was again raised by the member for Mallee, Dr Anne Webster
during her response to the second reading speech.[39]
Australian Greens
The Greens are yet to formally confirm their position on
the Bill.
Previously the New South Wales Greens have
proposed regulating vaping by undertaking such measures as: legalising nicotine vapes to reduce harms
and provide a pathway to quitting smoking; placing limits on nicotine levels;
requiring health warnings on all vaping products and making nicotine vapes
available to over 18s only. Recent reporting suggests that the Greens are not in favour of a prohibition
model, with Greens leader, Adam Bandt stating, ‘we take a harm minimisation
approach to issues concerning drugs … When it comes to other drugs, the Greens
don’t support a prohibition model. We think history has suggested that
prohibition models tend not to work’.
The Greens supported the inquiry into the legislation, stating concerns about
penalties for individuals and access to general practitioners (GPs) to receive
a prescription. In their additional comments to the Senate Committee’s
report into the Bill, the Greens raised concerns with aspects of the Bill and
stated that they ‘do not support the prohibition of vapes for adults and
instead will work towards a carefully regulated scheme that focuses on public
health outcomes, reducing harm and minimising the use of vaping products’. They
also flagged that they would move amendments to the Bill.[40]
Other parties
At this stage neither One Nation nor the Jacqui Lambie
Network appear to have commented on the Bill. Both One Nation and the Jacqui
Lambie Network previously voted
against a motion to regulate e-cigarette liquids.
Independents
The member for Kooyong, Dr Monique Ryan expressed
support for the legislation when
it was introduced and in a published opinion piece, noting that ‘[t]he government’s vaping legislation is important.
It’s possibly too late – and it’s going to be tough to enforce – but I
congratulate Mark Butler for having the courage to take on this scourge of an
industry’.[41]
The Member for Warringah, Zali
Steggall and the member for Indi, Helen
Haines also spoke in support of the Bill.[42]
Dr Sophie Scamps, member for
Mackellar, does not appear to have commented on the Bill, but has previously
called for stronger government action in relation
to vaping.[43]
Kate
Chaney, member for Curtin, Allegra
Spender, member for Wentworth and Kylea
Tink, member for North Sydney have all previously
spoken about strengthening regulations in relation to vaping.[44]
Position of major interest
groups
All Australian Health Ministers support the proposed Bill,
as stated in a Special
Communique on Vaping released on 19 April 2024:
All Australian Health Ministers have come together to declare
their support for coordinated action on vaping to protect young Australians.
…
Before the Federal Parliament there is currently world
leading legislation to ban the sale, supply, manufacture and commercial
possession of non-therapeutic vapes.
…
All Health Ministers have today urged the Australian
Parliament to pass the Albanese Government legislation, to ensure consistency
and coordinated action to protect the future generations of Australians.
When the Bill was introduced, a number of public health
organisations, including the Australian
Medical Association (AMA), the Royal
Australian College of General Practitioners (RACGP), the Cancer
Council, the Lung Foundation Australia and the Heart Foundation announced that they strongly supported the legislation and called
on MPs to support it. This support was reiterated in submissions
received to the Senate inquiry.
The Australian Medical Association stated:
The AMA strongly supports the Vaping Reforms Bill 2024. A
single consistent framework that applies nationally to reduce rates of vaping
and prevent long term adverse effects on population health is urgently
required. The Bill will reduce widespread availability of vapes by controlling
all levels of the supply chain … Importantly, the Government’s reforms will
support people to cease smoking and/or vaping by retaining access to
prescription vapes and making these more accessible to patients where
clinically appropriate through any GP. GPs are highly trained and experienced
in managing addiction with their patients and are experts in smoking cessation.
With new clinical guidelines, GPs are ready to continue helping patients to
quit.[45]
Cancer Council Australia supported reform in relation to
vaping:
Use of vaping products is rising exponentially among young
people. We have a rapidly closing window of opportunity to prevent a new
generation of Australians from becoming addicted to nicotine. With a Bill currently
before it that would enact strong measures to dramatically reduce supply of
vaping products within a matter of weeks, the Australian Parliament now has one
final chance to act before that window permanently slams shut.[46]
Support was also noted in submissions from the Australian
Council of State School Organisations, the Society of Hospital Pharmacists of
Australia and various public health researchers.
The Lung Foundation of Australia strongly supported the
Bill, but noted in its submission to the inquiry that it could be strengthened
by clarifying ‘personal possession versus commercial possession’:
Lung Foundation Australia does not want to see the
criminalisation of individuals for personal possession of e-cigarettes,
particularly for young people who have been targeted with these products.
…
Personal possession versus commercial possession should be
clearly defined so that law enforcement can focus efforts on those who sell
e-cigarettes outside of the regulated pathway.[47]
Concerns about legal
protections for individuals found to be in possession of vaping devices and
vaping accessories for personal use were also raised by the Australian
Alcohol and Other Drugs Council in its submission. It argued ‘the limited
personal use exemptions and significant criminal and civil penalties for
possession of vaping goods currently contained within the draft Bill risk a
range of unintended adverse consequences for people who use e-cigarettes who
consequently become engaged with law enforcement and justice systems’.[48]
The CEO of the Australian Association of Convenience
Stores (AACS), Theo Foukkare, has expressed
concern that the prescription model in the Bill may lead to an increase in
the black market supply of vaping products. In their submission to the Senate
Inquiry, the AACS reiterated concerns about illegal vapes and recommended a
regulatory based approach in which vapes are sold in the same way as alcohol
and tobacco.[49]
Concerns about the effect of the legislation on the black market have also been
raised in some media reports.[50]
A number of submissions to the inquiry were received from
individual vape retailers and individual vapers. Vape retailers generally
support a regulatory approach, expressing concern about losing their businesses
and an increase in illicit vapes under the proposed Bill. Individual vapers
expressed concerns about continued access to vaping products if the Bill is
passed, with a number noting that vaping had helped them quit, or substantially
reduce, cigarette smoking.
Financial implications
According to the Explanatory
Memorandum, ‘the Government will provide $82.0 million over four years from
2023–24 to support the vaping reforms, awareness raising and enforcement
activities’.[51]
This funding will include:
- $56.9
million over two years from 2023–24 to the TGA to support regulatory
development activities and
- $25.0
million over two years from 2023–24 to support the Australian Border Force’s
regulatory and enforcement activities.
This measure builds on the 2023–24 Budget
measure titled Vaping Regulation Reform and Smoking Cessation Package (see the Parliamentary
Library Budget Review which discusses these measures).[52]
Statement of Compatibility
with Human Rights
As required under Part 3 of the Human Rights
(Parliamentary Scrutiny) Act 2011, the Government has assessed the
Bill’s compatibility with the human rights and freedoms recognised or declared
in the international instruments listed in section 3 of that Act. The
Government considers that the Bill is compatible.[53]
Parliamentary Joint Committee
on Human Rights
The Parliamentary Joint Committee on Human Rights had no
comment on the Bill.[54]
Key provisions and issues
Changes to the definition of a ‘therapeutic good’
The Therapeutic Goods Act provides for standardised
controls on the manufacture, import, export, supply and use of therapeutic
goods in Australia. ‘Therapeutic good’ is defined
broadly in section 3 of the Therapeutic Goods Act as products for
use in humans in connection with:
- preventing,
diagnosing, curing or alleviating a disease, ailment, defect or injury
- influencing,
inhibiting or modifying a physiological process
- testing
the susceptibility of persons to a disease or ailment
- influencing,
controlling or preventing conception
- testing
for pregnancy.
This includes
products that are used as an ingredient or component in the manufacture of
therapeutic goods or used to replace or modify parts of the anatomy.
The Secretary of DHAC may
also make a declaration defining a good to be a therapeutic good where they
are satisfied that the good meets the definition of a therapeutic good.
Items 1 and 2 of the Bill will amend the definition
of therapeutic good to include a determination made under proposed
subsection 7AAA(1) which establishes a broad power for the Minister (in
practice their delegate) to declare goods to be therapeutic goods.
Before making such a determination the Minister must have
regard to whether:
- it
is likely that the specified goods, if not regulated under the Therapeutic
Goods Act, might harm the health of members of the public
- it
is appropriate in all the circumstances to apply the national system of
controls relating to the quality, safety, efficacy and performance of
therapeutic goods established by the Therapeutic Goods Act to regulate
the specified goods and
- the
kinds of risks from the specified goods to which members of the public might be
exposed could be more appropriately dealt with under another regulatory scheme.
Item 5 of the Bill makes a consequential amendment
to allow the Secretary to request information from a person who has imported
into Australia, or supplied in Australia, goods in relation to which the
Minister is considering making a determination under proposed subsection
7AAA(1) which can then be provided to the Minister.
As noted by the Explanatory Memorandum, these amendments
are not specifically related to the vaping reforms but rather allow for a
broader category of goods to fall within the TGA’s remit:
Allowing the definition of therapeutic goods to be broadened
in delegated legislation will enable rapid amendments to cater for novel and
unanticipated products that it is desired should be regulated as therapeutic
goods. Despite the broad definition of therapeutic goods, the importation,
manufacture, supply, export and advertising of goods in novel and unanticipated
forms that should be regulated as ‘therapeutic goods’ may not readily fit
within the current definition is still a possibility and may pose a risk to
public health. This is intended to be a broad power for the Minister to
specify any good to be a therapeutic good.[55]
[emphasis added]
The Scrutiny of Bills Committee’s general view is that
‘significant matters should be included in primary legislation unless a sound
justification for the use of delegated legislation is provided’.[56]
Given the existing definition of ‘therapeutic good’ is already broadly defined,
it is unclear why this broad power is required given the Parliament continues
to have the power to amend the Act to include goods which would not meet the
definition of a therapeutic good so that they fall within the regulatory remit
of the TGA.
Regulation of vaping goods
Key provisions
New definitions for ‘vaping goods’ and related terms
Part 2 of Schedule 1 inserts new Chapter 4A into
the Therapeutic Goods Act which provides for ‘the establishment and
maintenance of a national system of controls relating to the regulation of
vaping goods that are imported, manufactured, supplied in Australia or exported
from Australia’. The objects of the Act will also be amended to reflect this
additional purpose (see item 3).
The Bill will provide for new definitions for ‘vaping
goods’ and related terms (proposed section 41P). ‘Vaping goods’ includes
‘vaping substances’, ‘vaping accessories’, ‘vaping devices’, and goods
determined by the Minister to be vaping goods. The Minister will also have the
broad power to determine that goods are or are not vaping goods, including when
they are used, advertised or presented for use or supply in a particular way.
According to the Explanatory Memorandum, these definitions
would broadly mirror the definitions inserted into the Customs
(Prohibited Imports) Regulations 1956 by the Customs
Legislation Amendment (Vaping Goods) Regulations 2023 (CPI Regulations).[57]
However, some differences exist due to the ‘differing purposes’ of the Therapeutic
Goods Act and the CPI Regulations.[58]
New offences and civil penalty provisions relating to the
importation, manufacturing, supply and possession vaping goods
The Bill introduces offences and civil penalty provisions
that prohibit the importation, manufacturing, supply and possession of vaping
goods, subject to exceptions.[59]
According to the Explanatory Memorandum, the policy intent for introducing
these new provisions:
… is to ensure that vaping goods are only supplied through
the same supply chains that apply to goods containing substances included in
Schedule 4 to the Poisons Standard (prescription medicines). This will give
effect to the policy intention of ensuring that nicotine vapes are only
supplied to consumers under medical supervision with a prescription at a
pharmacy, subject to very limited exceptions.[60]
These new provisions have been drafted to include a tiered
penalty regime, with a new criminal fault-based offence, an additional offence
of strict liability, and a new civil offence.[61]
This is ‘an example of dual track regulation where the legislation gives
regulators a choice of responses in respect of the same physical conduct and a
range of enforcement options’:
[Other] [e]xamples include sections 19B and 19D of the TG
Act. Providing these options will enable the utilisation of a range of
regulatory tools, including the issuing of an infringement notice, commencing
civil penalty proceedings or criminal prosecution with a view to specifically
and generally deterring the proscribed conduct.[62]
The Explanatory Memorandum provides guidance on where a
criminal penalty would be pursued by the Commonwealth versus a civil penalty:
A criminal prosecution is considered to be a more appropriate
sanction where a contravention is deliberate, where fraud may be involved,
where the conduct demonstrates recklessness, where there is a serious pattern
of continuous intentional contraventions, or where conduct has endangered lives
or has caused death or serious injury.[63]
Table 1 – New
provisions in the Bill, along with the relevant penalties.
| Provision |
Offence |
Maximum penalty
(fault-based offence) |
Maximum penalty (strict
liability offence) |
Maximum penalty (civil
penalty) |
|
41Q
|
Importation of vaping goods
|
For an individual—imprisonment for 7 years and/or 5,000
penalty units.
For a body corporate—25,000 penalty units.
|
For an individual—200 penalty units.
For a body corporate—1,000 penalty units.
|
For an individual—7,000 penalty units.
For a body corporate—70,000 penalty units.
|
|
41QA
|
Manufacture of vaping goods
|
For an individual—imprisonment for 7 years and/or 5,000
penalty units.
For a body corporate—25,000 penalty units.
|
For an individual—200 penalty units.
For a body corporate—1,000 penalty units.
|
For an individual—7,000 penalty units.
For a body corporate—70,000 penalty units.
|
|
41QB
|
Supply of vaping goods
|
For an individual—imprisonment for 7 years and/or 5,000
penalty units.
For a body corporate—25,000 penalty units.
|
For an individual—200 penalty units.
For a body corporate—1,000 penalty units.
|
For an individual—7,000 penalty units.
For a body corporate—70,000 penalty units.
|
|
41QC
|
Possession of commercial quantity of vaping goods
|
For an individual—
At least the commercial quantity – 2 years
imprisonment/1,000 penalty units.
At least 100 times the commercial quantity – 4 years
imprisonment/3,000 penalty units.
At least 1,000 times the commercial quantity – 7 years
imprisonment/5,000 penalty units.
For a body corporate—
At least the commercial quantity – 5,000 penalty units.
At least 100 times the commercial quantity – 15,000
penalty units.
At least 1000 times the commercial quantity – 25,000
penalty units.
|
For an individual—
At least the commercial quantity – 120 penalty units.
At least 100 times the commercial quantity – 240 penalty
units.
At least 1,000 times the commercial quantity – 420
penalty units.
For a body corporate —
At least the commercial quantity – 600 penalty units.
At least 100 times the commercial quantity – 1,200
penalty units.
At least 1,000 times the commercial quantity – 2,100
penalty units.
|
For an individual—7,000 penalty units.
For a body corporate—70,000 penalty units.
|
|
41QD
|
Possession of less than commercial quantity of vaping
goods
|
For an individual—Imprisonment for 12 months and/or 500
penalty units.
For a body corporate—2,500 penalty units.
|
For an individual—60 penalty units.
For a body corporate—300 penalty units.
|
For an individual—1,000 penalty units.
For a body corporate—10,000 penalty units.
|
Source: Therapeutic Goods
and Other Legislation Amendment (Vaping Reforms) Bill 2024, Explanatory Memorandum, 47–48. The current value of a penalty unit is $313: Crimes (Amount of Penalty Unit) Instrument 2023.[64]
Each of these new offences are accompanied by relevant
exceptions which require the defendant to disprove, or raise evidence to
disprove, one or more elements of an offence. The Scrutiny of Bills Committee
has indicated that provisions imposing the burden of proof on the defendant
should be kept to a minimum, take into account the principles in the Commonwealth
Guide to Framing Offences, and that the Explanatory Memorandum should
describe the reason for placing the burden of proof on the defendant.[65]
With respect to this Bill, the Committee stated that while it:
… welcomes the inclusion of an explanation against each of
the reverse burden defences in the bill’s explanatory memorandum. However, the
committee’s view is that in most of these cases it is not apparent that the
matters are matters peculiarly within the defendant’s knowledge, or that
it would be significantly more difficult or costly for the prosecution to
establish the matters than for the defendant to establish them.[66]
For example, proposed subsection 41Q(5) provides
that it is an exception to the importation offences if the defendant can
provide that the importation of the vaping goods was not prohibited under the Customs Act
1901. In considering the justification for the reversal of the
evidential burden of proof provided by the Government, the Committee stated:
… that whether a person has been issued a permit or licence
by a government body administering the Customs Act 1901 is surely to be
knowledge retained, recorded and available to the prosecution by way of the
government body who retains these records. While it may be easier for a
defendant to produce this evidence than for the prosecution to disprove the
existence of such an approval, this does not equate, in the committee’s view,
to these matters being peculiarly within the knowledge of the defendant.[67]
Proposed section 41R provides that the Minister
may, by legislative instrument, determine that specified vaping goods, or a
specified class of vaping goods, may be supplied or possessed in Australia:
- by
a specified person, or a specified class of persons
- in
the circumstances (if any) specified in the determination
- subject
to the conditions (if any) specified in the determination.
The note to proposed section 41R provides that
conditions may, for example, relate to the value or amount of specified vaping
goods or the manner in which specified vaping goods may be supplied. The
Minister will also have the power under proposed section 41RA to
determine other indications (the specific therapeutic uses of the good) for
which vaping goods may be used which would allow them to be lawfully supplied
for this purpose. The Explanatory Memorandum states that ‘It is anticipated
that a determination would be made by the Minister under section 41RA that
would determine indications appropriate for medicinal cannabis vapes’.[68]
The Bill also introduces a consent scheme under proposed
section 41RC to allow persons who do not have a licence or authority under
the Therapeutic Goods Act or state and territory laws to lawfully
manufacture, possess or supply vaping goods. A person who has been granted a
consent under proposed section 41RC by the Secretary of DHAC will be
able to lawfully use the consent as a defence to the manufacturing, supply and
possession offences or civil penalty contraventions discussed above.
According to the Explanatory Memorandum, persons that can
be granted consent may include:
- persons
transporting vaping goods within a state or from one state to another
- persons
storing vaping goods for supply to a wholesaler or a pharmacist and
- pharmacists
compounding or manufacturing different vaping goods.[69]
However, as noted by the Scrutiny of Bills Committee,
‘there is no guidance on the face or the bill, nor in the explanatory
memorandum, as to what criteria may be considered by the secretary when
deciding whether to grant or refuse such an application, or in deciding which
conditions to impose, if any’.[70]
The Committee has sought the Minister’s advice on the need for this broad power
and what criteria may be considered by the Secretary in making such a decision.[71]
New restrictions on the advertising of vaping goods
The Bill also contains prohibitions on the advertising of
vaping goods in new Part 5-1A, which prohibit the direct and indirect
promotion of vaping goods subject to very limited exceptions, including patient
consultations, and authorisations given by the Secretary under proposed
section 42DZC.
The term ‘advertise’ is already defined in section 3 of
the Therapeutic Goods Act and includes, with respect to therapeutic
goods, any statement, pictorial representation, or design, however made,
that is intended, whether directly or indirectly, to promote the use or supply
of the goods. While the Explanatory Memorandum states that ‘that the term
advertise has a limited meaning and does not cover all communications in
relation to vapes’,[72]
according
to the TGA, 'any promotional activity for a therapeutic good is likely to
fall under the definition of an advertisement’.
As noted in the Explanatory Memorandum, this prohibition
would apply to ‘a broad range of media platforms, extending to social media and
other forms of advertising, promotion and sponsorship’.[73]
Proposed section 42DZF also introduces a broad
information gathering power which will allow the Secretary to request
information from a person responsible for the advertising of vaping goods. This
includes a broad power to require a person to provide ‘generic information’ relating
to vaping goods where that person may be responsible for disseminating, or for
causing the disseminating of, the information. The term generic information in
relation to vaping goods, includes any statement, pictorial representation or
design, however made, about the composition, properties or other
characteristics of the vaping goods, but does not include:
- an
advertisement about the goods
- generic
information included in an advertisement about the goods or
- bona
fide news.[74]
The Explanatory Memorandum does not provide any clarity on
what types of information would be considered ‘generic information’ and why
this power is required. Failure to comply with a request of the Secretary to
provide information is an offence, with a maximum penalty of 500 penalty units
for a fault-based liability offence and 100 penalty units for a strict
liability offence (proposed subsections 42DZG(1) and (2)). It is also an
offence to give false or misleading information in response to a request, with
a maximum penalty of 12 months imprisonment or 1,000 penalty units, or both,
for a fault-based liability offence and 100 penalty units for a strict
liability offence (proposed subsections 42DZG(3) and (4))
The Secretary will also be given the power to make
directions to the person responsible for the advertising of vaping goods or the
person apparently responsible for the dissemination of information, including
to cease or destroy the advertisement/generic information, make a retraction or
a correction, or recover any advertisement/generic information still in
circulation (proposed section 42DZK). Criminal and civil penalties will
apply where a person fails to comply with such a direction (proposed
sections 42DZL and 42DZM).
Key issues
Lack of clarity regarding what constitutes a ‘unit’ or a
‘commercial quantity’
The Bill amends the definitions in subsection 3(1) of the Therapeutic
Goods Act to provide that the quantity of a kind of vaping goods that will
be a ‘unit’, or a ‘commercial quantity’ will be the amount set out in
regulations.
These definitions are significant as they relate to
directly to whether a person has committed an offence (for example, proposed
sections 41QC (possessing at least the commercial quantity of vaping
goods)). As explained by the Scrutiny of Bills Committee, different penalties
also apply depending on the amount of the units above the commercial quantity
in contravention of an offence:
As an example, item 11 would insert proposed section 41Q into
the [Therapeutic Goods Act] which would create a new criminal offence,
an additional offence of strict liability, and a new civil offence, in relation
to the importation of vaping goods into Australia.
Proposed subsection 41Q(4) would provide that a person who
contravenes the civil offence provision in proposed subsection 41Q(3) would
commit a separate contravention in respect of each unit of vaping goods
imported by the person into Australia. While the committee generally only
comments in relation to criminal penalties, it is concerning that a significant
component of a civil offence will be left to delegated legislation.
In addition, proposed section 41QC provides for a range of
offences of possession where the person possesses differing amounts exceeding
the commercial quantity of vaping products, with higher penalties for the
larger amounts. As noted above, the prescription of the quantity of a kind of
vaping goods that would amount to a ‘commercial quantity’ will be left to
regulations, meaning that a significant component of the offences will be left
to delegated legislation.[75]
The Scrutiny of Bills Committee sought the Minister’s
advice on ‘why it is necessary and appropriate’ for these definitions to be
left to delegated legislation, noting the importance of these definitions to
the offence provisions proposed to be inserted by the Bill.[76]
Appropriateness of proposed penalties
As a general point, the Commonwealth
Guide to Framing Offences provides that ‘[a] penalty should be
consistent with penalties for existing offences of a similar kind or of a
similar seriousness. This should include a consideration of existing offences
within the legislative scheme and other comparable offences in Commonwealth
legislation such as the Criminal Code’.[77]
The Explanatory Memorandum to the Bill provides examples
with respect to the maximum penalties for comparable offences in the Therapeutic
Goods Act and other Commonwealth Acts, as set out in Table 2 below.
Table 2 –
Maximum penalties for comparable Commonwealth offences relating to therapeutic
goods and illicit tobacco
| Offence |
Description |
Maximum penalty –
imprisonment |
Maximum penalty –
financial |
|
s 19B(1) of the TG Act (aggravated offence)
|
unlawful importation, exportation, manufacture, supply
of therapeutic goods by the sponsor of the goods
|
5 years
|
4,000 penalty units
(or both)
|
|
s 19B(2) of the TG Act (fault-based offence)
|
unlawful importation, exportation, manufacture, supply
of therapeutic goods by the sponsor of the goods
|
12 months
|
1,000 penalty units
(or both)
|
|
s 19B(3) of the TG Act (strict liability)
|
unlawful importation, exportation, manufacture or supply
of therapeutic goods by the sponsor of the goods
|
Nil
|
100 penalty units
|
|
s 42DL(1) of the TG Act (aggravated offence)
|
unlawful advertisement of therapeutic goods
|
5 years
|
4,000 penalty units
(or both)
|
|
s 42DL(1) of the TG Act (fault-based
offence)
|
unlawful advertisement of therapeutic goods
|
12 months
|
1,000 penalty units
(or both)
|
|
s 42DL(1) of the TG Act (strict liability)
|
unlawful advertisement of therapeutic goods
|
Nil
|
100 penalty units
|
|
s 42E of the TG Act (fault-based offence)
|
importation, exportation, manufacture or supply of
counterfeit therapeutic goods
|
7 years
|
2,000 penalty units
(or both)
|
|
s 308-10 of Schedule 1 to the Taxation Administration
Act 1953
|
possession of tobacco (500 kilograms or
above)—reasonable suspicion offence
|
5 years
|
1,000 penalty units
or both 5 years and the greater of 1,000 penalty units
or 5 times the excise duty
|
|
s 308-15 of Schedule 1 to the Taxation Administration
Act 1953
|
possession of tobacco (100 kilograms or
above)—reasonable suspicion offence
|
2 years
|
500 penalty units
or both 2 years and the greater of 5 times the excise
duty
|
|
s 308-20 of Schedule 1 to the Taxation Administration
Act 1953
|
possession of tobacco
(5 kg or above)—reasonable suspicion offence
|
Nil
|
200 penalty units or the greater of 5 times the excise
duty
|
|
s 320.2 of the Criminal Code
|
importation of psychoactive substances
|
5 years
|
300 penalty units
(or both)
|
The Explanatory Memorandum states that in the context of
the offences set out in the table above:
… the maximum penalties for offences relating to the
importation, manufacture and supply of vaping goods are considered appropriate,
being 7 years imprisonment for fault-based offences and 200 penalty units for
strict liability offences. Equally, the sliding scale maximum penalties for
offences relating to the possession of vaping goods are also appropriate.
Depending on the quantity of vaping goods possessed, a maximum penalty of 7
years, 5 years, 2 years or 12 months will apply for fault-based offences and
420 penalty units, 240 penalty units, 120 penalty units or 60 penalty units
will apply for strict liability offences.[78]
However, the only offence listed in the table above that
provides for a term of 7 years imprisonment for a fault-based offence is
section 42E of the Therapeutic Goods Act, with the criminal penalty
being 2,000 penalty units (or both). Section 42E makes it an offence to import,
export, manufacture or supply counterfeit
therapeutic goods, which can include over-the-counter products (like
paracetamol and ibuprofen) to life-saving medicines (such as AIDS, cancer and
heart medicines). As noted by the TGA, 'using counterfeit medicines or medical
devices carries a high risk of unexpected or potentially serious reactions’ and
is considered to be a ‘serious threat to public health’.[79]
The maximum criminal penalties for offences relating to the importation,
manufacture and supply of vaping goods are 7 years imprisonment and/or 5,000
penalty units which is a higher financial penalty than what is set out in
section 42E. The inclusion of terms of imprisonment for the maximum penalty for
fault-based vaping offences seems disproportionate to the existing penalties in
the Public
Health (Tobacco and Other Products) Act 2023 for dealing with and
possessing permanently banned tobacco products (sections 127 and 128).
With respect to the proposed possession offences,[80]
it is difficult to compare these to existing possession offences in
Commonwealth legislation given the lack of detail regarding what will
constitute a ‘unit’ or a ‘commercial quantity’.
Ability for individuals to be prosecuted for possession of
vapes
The Government has stated that in relation to possession,
‘the intent is not to criminalise or otherwise prohibit the personal possession
of vaping goods. It is only commercial possession that is intended to be
prohibited or possession other than for personal use’.[81]
This is somewhat reflected in the inclusion of proposed subsection 41QD(9)
which provides an exception for personal use or possession on behalf of another
person where a person has committed the offence of possessing less than the
commercial quantity of vaping goods. The Explanatory Memorandum states that
this exception:
… has been included to protect persons who may be possessing
vaping goods on behalf of someone who requires the vaping goods in connection
with treatment for smoking cessation or the management of nicotine dependence,
including carers and nurses in a hospital or nursing home setting.[82]
However, the person bears the evidential burden of proving
that the vapes were for personal use/possession on behalf of another person. Proposed
section 41QC (where the person is in possession of a commercial quantity of
vaping goods) does not include a similar exception.
Ability for children to be prosecuted for vaping offences
Under section 4M of the Crimes Act
1914, the minimum age of criminal responsibility for Commonwealth
offences is 10 years of age. Subsection 4N(1) provides that a child aged 10
years or more but under 14 years old can only be liable for an offence against
a law of the Commonwealth if the child knows that his or her conduct is wrong,
and subsection 4N(2) specifies that ‘whether a child knows that his or her
conduct is wrong is one of fact’. The burden of proving that the child
has sufficient capacity to know that the act/omission was one they
ought not to do or make is on the prosecution, using evidence such as medical
records, school reports, any police interviews with the child et cetera.[83]
The most relevant offences with respect to children in the
Bill are proposed sections 41QC (possessing at least the commercial
quantity of vaping goods) and 41QD (possessing less than the commercial
quantity of vaping goods).
New enforcement powers for therapeutic goods
The Bill will also expand the existing enforcement powers
applying to the regulation of therapeutic goods which are currently set out in Chapter
5A of the Therapeutic Goods Act, as well as allowing the Secretary to
delegate their functions under Chapter 5A, proposed section 52AAA
(forfeiture of things seized under search warrant) or proposed section 52AAB
(return of retention of thing declared not to be forfeited) to a relevant
state/territory officer (proposed subsection 57(1A)).
New power to issue enforceable directions
The Bill will insert new Part 5A-5 into Chapter 5A which
provides for the broad power of the Secretary to give enforceable directions.
Proposed section 42YT permits the Secretary to make
written directions to a person requiring the person to do specified things in
relation to particular goods if the Secretary believes, on reasonable grounds:
- a
person is not complying with the Therapeutic Goods Act in relation to
particular goods and
- it
is necessary to exercise this power to protect the health and safety of humans.
This power allows directions to be made requiring one or
more of the following:
- to
relabel or label goods in compliance with the requirements under the Therapeutic
Goods Act
- to
repackage goods in compliance with the requirements under the Therapeutic
Goods Act or
- to
destroy or dispose of goods at their own cost, including by delivering the
goods to specific service providers who will dispose of the goods responsibly.
In complying with these directions, the person will bear
the expense and criminal and civil penalties will apply where a person fails to
comply with the directions. According to the Explanatory Memorandum, these
powers are required ‘to manage waste issues associated with enforcement of
vaping laws, but also to give the Secretary a broad range of compliance and
enforcement tools to manage unlawful vapes and therapeutic goods generally’.[84]
Forfeiture and destruction of goods
The Bill will also insert new Part 6-2A into the Therapeutic
Goods Act which provides for the forfeiture of things seized under search
warrants in certain circumstances.
Currently under section 54 of the Therapeutic Goods Act
forfeiture orders can only be obtained following a criminal conviction, an
order under section 19B of the Crimes Act 1914, or a finding by a court
that the person has contravened a civil penalty provision.[85]
Under section 48H of the Therapeutic Goods Act, seized goods may be
required to be returned to the owner if they are no longer needed for
evidentiary purposes, regardless of whether it is lawful for the owner to
possess or deal with the goods.[86]
Proposed subsection 52AAA(1) provides that where a
thing has been seized under a warrant issued under section 50 of the Therapeutic
Goods Act and the Secretary believes on reasonable grounds:
- that
the thing has been imported, manufactured or supplied or been in the
possession, custody or control of a person, in contravention of the Therapeutic
Goods Act or
- an
instrument made or requirement under the Therapeutic Goods Act has not
been complied with
then the thing is forfeited to the Commonwealth. Upon the
thing being condemned as forfeited to the Commonwealth, the Secretary may
retain the thing for the purposes of proceedings in respect of which the thing
may afford evidence or dispose of the thing in a manner in which the Secretary
directs.
The owner of the thing, or person who had possession,
custody or control of the thing, may commence proceedings for a declaration
that the thing is not forfeited to the Commonwealth (proposed subsection
52AAA(4)). Proposed section 52AAB provides for the return or
retention of a thing that is declared not to be forfeited to the Commonwealth
and allows the Secretary to impose terms and conditions on the return of things
as the Secretary sees fit.
According to the Explanatory Memorandum, these amendments
‘will enable the TGA to disrupt the trafficking of unlawful goods more
efficiently, as well as address potential storage and destruction issues where
large quantities of unlawful vapes are seized’.[87]
However, they will not be limited to the seizure and destruction of vaping
products.
Expanded power to issue warrants
Currently sections 49 and 50 of the Therapeutic Goods
Act require warrants to be issued by a magistrate. Item 58 of the
Bill will amend the definition of ‘issuing officer’ in subsection 3(1) of the
Act to allow court registrars and other legislative officers of a court of a
state or territory to also issue warrants.
According to the Explanatory Memorandum:
The TGA often experiences operational [delays] when seeking
timely access to magistrates to issue warrants. This is problematic when there
is urgency in the obtaining and execution of a warrant. Consequently, these
amendments extend the authority to issue monitoring, offence and civil penalty
related warrants in relation to premises to court registrars and other
legislative officers of a court of a state or territory.[88]
Access to vapes for smoking cessation purposes
Effects of vapes as smoking cessation aids
The most efficient means of finding quality scientific
research on healthcare interventions is through meta-analyses or systematic
reviews of articles published in peer-reviewed journals. Of these, the Cochrane
Reviews of the evidence on healthcare interventions and summaries of the
findings are generally recognised to be the gold
standard.
Several Cochrane Reviews of vapes as a smoking cessation
tool have been conducted, the
most recent of which incorporates evidence published up to the 1st of July
2023.[89]
This review prioritised randomised controlled trials but
also included studies in which all participants received vape treatment and had
their health monitored, to evaluate the health impacts of vapes. The review was
primarily concerned with the question of how many people using vapes as smoking
cessation devices stopped smoking for at least 6 months and how many trial
participants had unwanted effects. Vapes were compared with a range of other
methods of quitting smoking.
Based on an analysis of 88 studies, which included 27,235
adults who smoked, the review found that people were more likely to stop
smoking for at least 6 months using nicotine vapes than using nicotine
replacement therapy or vapes without nicotine. The review also found that
nicotine vapes may work better than no support, or behavioural support alone,
and that they may not be associated with serious unwanted effects.
These findings are qualified by the review’s authors. They
note that the results are based on a few studies for most outcomes and that for
some outcomes the data varied widely. They also stress that more studies are
needed to confirm whether nicotine vapes are more effective than non-nicotine
vapes, and that most results for unwanted effects ‘could change when more
evidence becomes available’.
In light of the still limited population-level evidence
regarding the effectiveness of vapes as smoking cessation aids, the products are
used as a second-line treatment in Australia. As such, they may be
recommended by general practitioners (GPs) when smoking cessation with
first-line therapy (behavioural support combined with TGA-approved
pharmacotherapy) has failed.
Numbers using vapes to quit smoking
Judging by NDSHS
data for 2022–23, 21.5% of vape users aged 14 and older are using the
products to help them quit smoking. This proportion is less than that in the
2019 survey (32.5%). Other reasons for the use of vapes include people thinking
they are less harmful than regular cigarettes (15.8%), to try to cut down on
the number of cigarettes smoked (15.6%), and to try to stop going back to
smoking regular cigarettes (13.9%).[90]
Under existing laws, a valid medical prescription is
required to access nicotine-containing vapes or nicotine containing vapes. However,
based on figures
cited by the TGA in its impact assessment of options to address the public
health problem posed by vapes, relatively few vapers are procuring them by
means of a prescription. Several recent surveys suggest that between 3% and 12%
of all vapers had a prescription.[91]
Similarly, based on numbers of authorised providers and data on prescriptions,
the TGA has observed that only a very small proportion of vape users seek
prescriptions.[92]
NDSHS data for 2022–23 indicate that only 0.3% of current vapers obtained their
vapes from a pharmacy or chemist.[93]
As
the TGA sees it this is less than ideal as it leaves people using directly
sourced vapes as a means to quit smoking without the support of a medical
practitioner:
Ensuring that vapes are only accessed with medical
supervision provides an opportunity for consumers to receive appropriate advice
from a health professional on the risks associated with their use and the
benefits of smoking cessation. Medical supervision also enables close
monitoring of emerging and ongoing health harms that may be associated with
vaping.[94]
That people are currently bypassing official channels to
source vapes for smoking cessation purposes is perhaps unsurprising for a
number of reasons.
Availability and cost
of illicit nicotine vapes
Despite it being an offence in all states/territories to supply
nicotine vapes to consumers without a prescription, nicotine containing vapes
are readily available. Illicit nicotine containing vapes are also typically
cheaper than those purchased through a pharmacist. According to the TGA, ‘[t]he
ease of access to, and low cost of, illegal supply can make this a preferred
option, even if an adult has obtained a legitimate prescription’.[95]
Until such time as vapes are registered as a prescription
medicine on the Australian Therapeutic Register of Therapeutic Goods (ARTG) and
listed on the Pharmaceutical Benefits Scheme (PBS)—and perhaps even then—they
will remain more expensive than those purchased through other means.
The TGA has noted that ‘the cost of vapes sourced solely
through a pharmacy may be significantly higher than those sourced through most
current means’.[96]
There is a risk this situation could worsen under the changed arrangements,
with additional costs associated with enhanced manufacturing standards being
passed on to consumers, and pharmacies possibly exploiting a monopoly on
supply.[97]
The costs associated with obtaining a prescription are also likely to increase
the expense of therapeutic vapes.
The cost of obtaining a prescription was noted in the Impact
Analysis which found that the average cost per year for consumers
(including GP and pharmacy visits) and the system costs over ten years, would
be $52.1 million per year.[98]
Other factors that limit the supply of vapes for
therapeutic purposes could also translate into higher prices for these
products.[99]
The cost of therapeutic vapes and pharmaceutical grade vape fluid could be
expected to decrease over time if the market for the products were to increase.
However, the TGA anticipates that this will not be the case, with the number of
consumers declining over time ‘as consumers respond to public health messaging
and advice from medical practitioners regarding the danger of vaping’.[100]
The generally higher cost of therapeutic vapes could
result in an increase in black market sales, and, more alarmingly, an increase
in cigarette smoking.
The TGA has acknowledged the potential for changes to
regulatory settings to further increase black market activity. It has also
noted that ‘if access to nicotine-containing vapes were to be significantly
restricted there is a risk that Australians addicted to nicotine may seek
alternative products to meet cravings, such as tobacco’.[101]
The TGA has outlined a range of measures calculated to
manage these potential risks. In the case of a possible increase in tobacco use
due to consumers being unable to source therapeutic vapes, the TGA has observed
that alternative smoking cessation aids are available. It has also suggested
that the risk can be reduced ‘by ensuring ease of supply for legitimate use and
access ... [and] ‘implementing preventive public health campaigns focused on
vaping and smoking cessation and increasing and improving vaping and smoking
cessation support’.[102]
Recently
introduced measures that tighten tobacco plain packaging requirements
should also help to reduce the possibility of a shift to cigarette smoking.[103]
Access to authorised
prescribers
Another impediment to the greater use of therapeutic vapes
is the limited number of authorised prescribers. Currently, many GPs are likely
to be reluctant to prescribe vapes because, as noted above, these products have
yet to be evaluated by the TGA and registered on the ARTG as smoking cessation
devices. In the absence of such approval, and with outstanding concerns
regarding evidence of vapes’ safety and efficacy as smoking cessation devices,
GPs may simply be erring on the side of caution and recommending the use of
first-line smoking cessation therapies which have an established safety and
efficacy profile.
Enabling nurse practitioners as well as medical
practitioners to supply therapeutic vapes and vaping goods may or may not
improve consumer access.
Based
on its modelling the TGA estimates that following the shift to a
prescription only model 450,000 vapers will move from illegal to legal
channels.[104]
Once the regulatory changes are in place the TGA estimates that the number of
authorised providers will increase by 10% in the first year and 2% per year
thereafter. The initial boost in numbers is anticipated to be a result of ‘more
confidence by prescribers in the new regulatory regime and pressure from
patients’.[105]
If the move to a prescription model that the Bill would enable proves
unsuccessful, with people shifting to the illicit market or smoking, Wayne Hall,
an Emeritus Professor at the National Centre for Youth Substance Abuse, has suggested
an alternative approach of allowing the sale of approved vapes to adult smokers
through licensed retailers under tighter regulations than apply to cigarettes.[106]
This ‘pragmatic
consumer model’ would retain most of the restrictions proposed in the Bill
and already implemented but would not require a prescription for vapes or
restrict dispensing to pharmacies.[107]
Smoking cessation support for under 18s
One of the main purposes of the Bill is to reduce vaping
prevalence in the community, particularly among young people. The Explanatory Memorandum
to the Bill states that ‘[i]n parallel to the introduction of the Bill,
cessation services in Australia will be enhanced to ensure support for people
who seek assistance to stop vaping’.[108]
As noted in the NDSHS, between 2019 and 2022–23 vaping
increased among people aged 14–17 from 1.8% to 9.7%; however, it is unclear
what assistance will be provided to young people who may be addicted to vaping,
or those seeking to quit or taper down. The DHAC submission to the Senate
inquiry outlines a number of cessation support programs announced in the 2023–24
Budget including funding for ‘a national campaign to target young people, their
parents, carers and communities, as well as a stream of activities dedicated to
reaching teachers and the education sector’,[109]
but this does not directly address how under 18s who are already vaping will be
supported.
Provisional guidance from the RACGP on prescribing vapes
for smoking cessation, provisional guidelines (noted above),
states the following regarding persons under 18:
[Nicotine vaping
products] NVPs are not currently recommended as a smoking cessation aid for
people under 18 years of age as to date there have been no studies of
effectiveness and safety in this age group. For further information about
smoking cessation in this group, refer to Adolescents
and other young people. For advice on assisting adolescents who are already
vaping see the section on vaping cessation.[110]
However, in a webinar held in conjunction with the TGA,
the RACGP noted that there was uncertainty around how to best treat young
people who are vaping due to a lack of evidence:
The prevalence of young people who vape (and may have never
previously smoked) is rising however there is a currently a lack of evidence on
the effectiveness and safety of medicines for smoking cessation in this group.
Young people who are nicotine dependent through vaping may
benefit by using a therapeutic vape to manage nicotine dependence for a limited
period with the intention to stop vaping, but again there is a lack of research
on effectiveness and safety.
There are differences across jurisdictions about the legality
of supplying a young person with a therapeutic vape even if it is on
prescription. Currently information on this is not easily available and is
likely to change over time.[111]